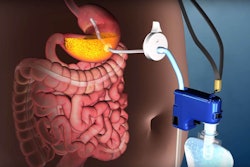
This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
The final guidance allows manufacturers to use the ISO 10993-1 standard when assessing the potential biological response of the medical devices and materials that come into direct or indirect contact with the human body. Specifically, the recommendations in this final guidance clarify and update how medical device developers may use ISO 10993-1 standard in their premarket submission to determine the potential for an unacceptable adverse biological response resulting from contact of a medical device with the body. This final guidance replaces the Office of Device Evaluation (ODE) Blue Book Memorandum #G95-1 (1995), entitled “Use of International Standard ISO-10993, ‘Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing.”
Additionally, this guidance highlights the concept that a biocompatibility assessment should begin with a risk-based approach instead of immediately considering new biocompatibility testing. If the risk assessment indicates that biocompatibility testing is warranted, new test-specific recommendations are included in this guidance to address common deficiencies identified in premarket submissions. Implementation of the recommendations in this guidance will occur on September 14, 2016.
For more information, click here