Catalent Pharma Solutions, a world industry leader in drug development and advanced delivery technologies, today announced the establishment of a global alliance with PAREXEL International Corporation to help streamline the clinical trial supply process. The result of years of collaboration, the alliance will provide a seamless, fully integrated service offering to ensure the timely and efficient delivery of clinical trial materials.
“As clinical trials become more complex, we feel it is imperative to help sponsors devise supply strategies and organize consistent manufacturing, packaging, and delivery of trial-related supplies to locations around the world,” stated Gerry Hepburn, Chief Operating Officer, Vice President and General Manager of Clinical Supply Services at Catalent. “PAREXEL, with its extensive and unparalleled track record in clinical development, is the ideal partner for Catalent in this alliance, which willenable our customers to compete more effectively in the global marketplace.”
“One of the most important steps in creating a strong, efficient clinical trial material supply chain is selecting a partner with innovative clinical packaging solutions and established global operations to oversee and coordinate all aspects of each critical supply chain stage,” commented Kurt Norris, Corporate Vice President, Clinical Logistics Services, PAREXEL. “In establishing this alliance with Catalent, we have chosen a partner that is uniquely positioned to help biopharmaceutical companies reach critical development milestones through more efficient delivery of clinical trial supplies.”
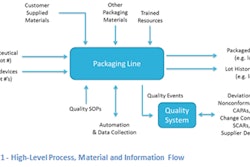
The alliance will provide fully integrated services and technologies across the entire clinical trial material supply chain spectrum, supporting all supply aspects in clinical development programs including:
• Planning and development of supply strategies
• Material supply and demand forecasting
• Central and local clinical trial and ancillary material sourcing
• Innovative clinical manufacturing and leading clinical trial packaging solutions
• Worldwide regulatory and import/export management
• Global and local storage as well as distribution to trial sites
• Depot, site and patient inventory management
• Material return and destruction/disposition
The establishment of this alliance follows Catalent's February 2012 acquisition of the Clinical Trial Supplies business of Aptuit Inc., which transformed Catalentinto the #2 global provider of clinical supply solutions, and enhanced Catalent's leading global position in development solutions and advanced delivery technologies for drugs, biologics, and consumer health products.
Companies in this press-release