Despite the Drug Supply Chain and Security Act, the efforts of Interpol’s Operation Pangea, and the efforts of pharmaceutical manufacturers, “the global counterfeit drug trade is a booming business,” says the Aug. 31 CNN.com report, “Deadly fake Viagra: Online pharmacies suspected of selling counterfeit drugs.”
The article notes, “One of the main challenges of curbing the counterfeit trade is, ‘a lack of universal laws that criminalize counterfeit medicine,’” quoting John Clark, former Deputy Assistant Secretary for the U.S. Immigration and Customs Enforcement agency and now VP and Chief Security Officer, Pfizer Global Security. Clark tells CNN, “Right now it is handled differently country by country. There needs to be global governance on this issue.”
Pfizer’s website addresses several factors leading to increased counterfeit medicines: “Included among them are the growing involvement in the medicine supply chain of under-regulated wholesalers and repackagers, the proliferation of Internet pharmacies, advancements in technology that make it easier for criminals to make counterfeit medicines, the increased importation of medicines from Canada and other countries, and the relatively small risk and penalty faced by counterfeiters.”
The site notes that in 2013, “authorities from 49 countries seized more than 11.8 million tablets, capsules, and vials of counterfeit Pfizer medicines. Many of the raids resulted from leads developed by Pfizer Global Security.”
Pfizer provides web visitors with a comprehensive list of additional resources related to counterfeiting and importation.
FDA examples
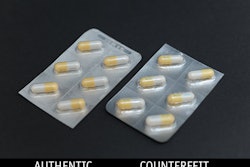
A Sept. 1 look at the FDA’s website offered numerous examples of drug counterfeits, including the following two:
• Counterfeit version of Botox found in the U.S. (4-16-2015).
In this instance both the outer carton and the vial were counterfeit as the vial was missing the lot number, the outer carton did not have any entries next to the LOT:MFG: EXP:, and both the outer carton and vial incorrectly displayed the medication’s active ingredient.
• Counterfeit versions of Cialis tablets entering the U.S.
Here there were differences on the bottle label between the counterfeit product and Eli Lilly’s authentic product. The counterfeit version did not include an NDC number on the bottle front, tablet strength details, while it was printed in different patterns and colors, complete misspellings, even in the product’s name.
Criminals follow the money, and as more advanced medications with higher price tags reach the market, they appeal to counterfeiters, particularly since their effort reportedly carries modest risks and penalties.
Pfizer’s point about advancements in technology making it easier for counterfeiters is unnerving. That said, the battle on the front lines of serialization rages forward. And it’s not being fought solely by manufacturers, but throughout the supply chain.
Healthcare Packaging’s recent cover story detailed how Domino, its supplier partners, and its pharmaceutical manufacturer customers were addressing DSCSA/DQSA through interconnecting packaging equipment, materials, and components.
In an upcoming Healthcare Packaging story, you’ll read how a global pharmaceutical contract packager is investing millions of dollars in packaging equipment, software, and human resources for serialization efforts within its facilities and for lines to help its pharmaceutical/biopharmaceutical customers comply with federal serialization regulations.
Yes, the counterfeiting battle continues, and it sometimes appears to be a losing effort, but it’s reassuring to see how the packaging community is making progress in ensuring that patients receive legitimate medications, and making progress toward ultimately winning the war.