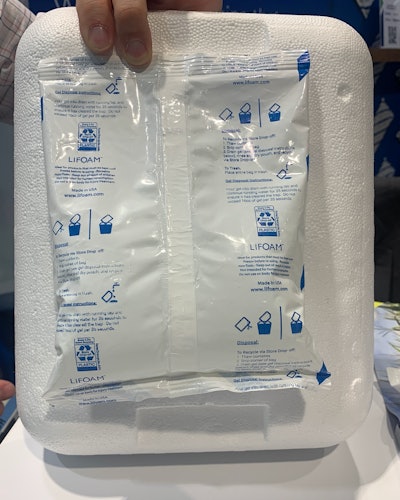
In temperature-controlled shipping sustainability, Lifoam Industries, LLC, a division of LifeMade Products LLC, a Jadex Inc. company, has launched its recyclable Propak gel bags this year. They’ve taken their widely established standard Propak gels and placed them in a recyclable film. Applications for the gel bags range from consumer food deliveries to pharmaceutical shipping—any area using water-based gels.
“We've seen the trend towards sustainability and know that these are going to a lot of home users who want to dispose of gel packs responsibly after their deliveries,” Alex Arabea, senior brand manager, said, “My typical example is you get a meal kit once and say, ‘Cool. A free gel pack for my kid’s lunch.’ Then you get a second one. ‘Oh nice, another one.’ You get a third, and ask, ‘What do I do with this?’ And you're wondering how to responsibly dispose of it.”
Unlike some gels that cause clogs when poured down drains, the user is able pour the liquid from recyclable Propak gel bags down their home drain without clogs. “It’s perfectly safe to put this gel down the drain. We have worked with water and wastewater treatment organizations and conducted testing to confirm that it won't harm anything down the line,” he explained.
Disposal and sustainability
The bag has disposal instructions in clear print on the back, using the How2Recycle symbol that consumers are familiar with. Empty Propak gel film is recycled via store drop-off, as curbside recyclable films for this application are not readily available. “Consumers see that How2Recycle symbol on everything from water bottles, food packaging, and more, so it helps with that consumer education piece. They’ll know how to properly dispose of these products and take advantage of the sustainability that we built in, without them having to do extra research on their own,” Arabea noted.
They have also updated branding on the bags. “Our gel bags look a little bit different than they have in the past—this is a move towards sustainability. We've done all the testing to say that there's no requalification necessarily, there's no negative impact. Just a sustainable wrapper around the same gel,” he said.