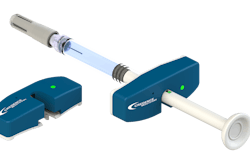
If you’ve been following the news, you’ve noticed that each country affected by coronavirus is scrambling to contain it. A recent article from The Verge covered the FDA’s effort to curb the spread of the deadly disease. Last week, the FDA issued an expedited approval for a coronavirus diagnostic test, allowing state health labs to use it. Prior to that, all samples from suspected cases in the country had to be sent to the Centers for Disease Control and Prevention for testing.