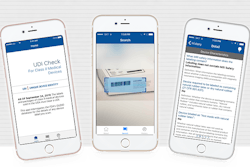
As part of September 2016’s FDA medical device regulations, the labels and packages of Class II devices must bear a UDI, and data must be submitted to the global unique device identification database (GUDID) database. Authentag, an information and technology services company, has created an app for checking medical device identification information.
Authentag Introduces UDI Scanning App
Medical device manufacturers must ensure that the FDA GUDID database is kept up to date with unique device information. A new app allows users to obtain GUDID information by scanning the device's barcode.
Jan 25, 2017
List: Digitalization Companies From PACK EXPO
Looking for CPG-focused digital transformation solutions? Download our editor-curated list from PACK EXPO featuring top companies offering warehouse management, ERP, digital twin, and MES software with supply chain visibility and analytics capabilities—all tailored specifically for CPG operations.
Download Now
Sustainable Healthcare Packaging Solutions That Work
Industry leaders share proven strategies for reducing packaging emissions by up to 70% while meeting safety and regulatory requirements.
Read More
Downloads