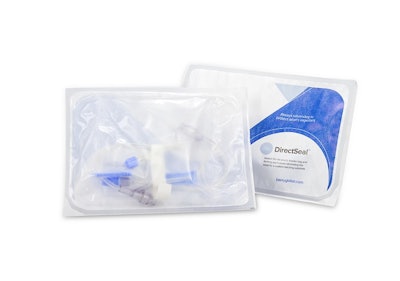
Berry Global Inc. has released two new medical packaging products:
DuraMed is a patented system for form-fill-seal packages, header bags, and peel pouches. The material, which can be used for sterile or non-sterile applications, was designed for breathability, barrier, clean peel, and high resistance to tear and puncture, and is compatible with ETO sterilization.
DirectSeal is a film with sealant technology, which was designed to eliminate the need for a matching coated substrate for applications including form-fill-seal, header bags, and peel pouches.The material, which can be used with DuraMed, provides consistent seal across temperatures with uncoated paper and flash spun HDPE.






















