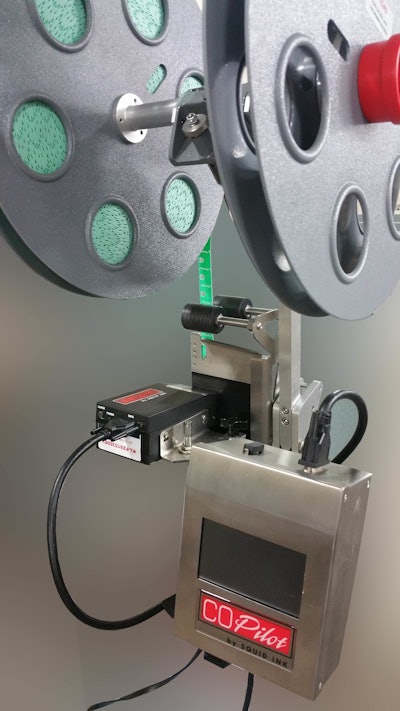
Squid Ink Manufacturing, in partnership with Kwik Lok Corp. announces the new 905A CoPilot UV LED printer, which is designed to be attached to the Kwik Lok 872 Automatic Bag Closing Machine, utilizing the CoPilot printer and UV LED Curing System built by Squid Ink Manufacturing.
The new system offers immediate cure time and clean, crisp codes on the Kwik Lok bag closure, resulting in a low maintenance system with low overall cost of ownership.
The CoPilot UV LED curing and printing system offers accurate and consistent UV curing across a variety of commercial and industrial applications. UV cure ink is a non-drying, minimal maintenance ink cured by LED light. This instant LED curing ink is impervious to environmental factors such as sunlight, water, moisture, temperature, and contains virtually no VOC’s.
The Kwik Lok® 872 Automatic Bag Closing Machine enables the packager to close and label bagged packages with one machine at speeds up to 105/min. The system will close a wide range of product size variations. Striplok® closures are available in many closure opening sizes to accommodate a large number of bag widths and film thicknesses. The 872 features an operating panel for speed control (0 to 105 bags/min), a shift counter, lifetime counter, and a manual cycle button.





















