The RadioGenix system is used by radiopharmacies and nuclear medicine departments for the diagnosis and treatment of various medical conditions. There are some 380 of these specialty commercial radiopharmacies in operation in the U.S.
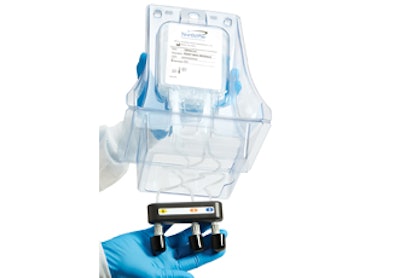
For Madison, WI-based NorthStar Medical Radioisotopes LLC, the package holds and protects three separate bags filled with liquid reagents—one of which is caustic—and its tubing during shipping, handling, and storage. Packaging also includes an RFID chip “all in one elegant, self-contained, tamper-proof package,” says Edmond J. Fennell, NorthStar’s VP, Business Development. “The clear plastic allows visual inspection without the need to open the package.”
He adds, “The packaging serves as a ‘reagent pod’ that holds all the reagents and is suspended above the RadioGenix Generator System and allows the user to effortlessly connect the reagent tubing to the system. An additional, but very important feature is that it’s easy to open and dispose. All in all, it’s a very efficient, practical solution for this aspect of the system.”
The package is designed and thermoformed by Barger, Placon’s medical division. It is also an integral part of the technetium-99m generation process used in millions of medical diagnostics procedures every year, making it “the most commonly used medical radioisotope,” according to Wikipedia.
Tubing connects the reagent bags held inside the lower cavity of the package to NorthStar’s proprietary new medical instrument. Through this connection, the liquid reagents are consumed in a chromatographic process to produce critical and commonly used isotopes for use in medical diagnostic imaging procedures.
The packaging’s handling, safety, ease of use, convenience and functionality helped it earn honors as an Institute of Packaging Professionals’ 2016 AmeriStar award winner in the medical device category.
Meeting packaging challenges
“The packaging solves a number of critical challenges for our engineers, and by extension, our customers,” says Fennell.
He explains, however, the system is not yet in commercial use. “NorthStar is working to gain FDA approval of the system with a realistic target date in sight. The variant of the current system NorthStar is developing will replace the existing Mo99/Tc99m generators offered to the nuclear medicine community. These existing systems are adequate but lack today’s demand for ‘smart-technology’ that provides traceability and safety features taken for granted in other areas of healthcare.”
The overall size of the trifold reagent module is 7.26 x 5.61 x 10.79 in. The three sections of the package are connected by two living hinges, allowing the unit to be folded and securely closed. The outer sections of the packaging form contoured wells for 50-mL single-use bioprocess bags, while the middle section forms a tray that houses coiled drain lines from the bags, oriented and secured by a keyed RFID chip assembly. The bottom of the center section has a perforated panel that allows quick access to the gang of couplers and RFID key.
NorthStar developed a concept for packaging, providing a safe, intuitive single module for the reagents with thermoforming in mind, Fennell says. “NorthStar did not have an approved thermoform supplier, so medical device thermoformers throughout the Midwest were evaluated and Barger quickly came to the forefront with responsive and collaborative customer service as well as competitive pricing,” he notes.
The packaging process
Barger thermoforms, cuts and trims all parts from 0.04 PETG with a slight transparent blue tint. Barger provides the parts nested and double-bagged for further assembly in a cleanroom environment.
Bioprocess bag filling is outsourced, with NorthStar assembling the filled bags into the trifold packaging. It also assembles and programs the RFID keys. New equipment was required to ultrasonically seal the bag inlet tubing post-fill. NorthStar worked with Branson Ultrasonics, a business of Emerson Industrial Automation to define the fixturing and welding process for consistently sealing the bag tubes.
Preservation Solutions Inc. worked with NorthStar to define the needs of the filling process and ensure the custom bag design met their needs for aseptic filling. Saint Gobain worked with NorthStar in the design of the bag assembly. It ensures material compatibility with caustic reagent chemicals, a robust design and a tightly controlled manufacturing process. Saint Gobain supplies all reagent bags assembled and sterile to Preservation Solutions for filling.
Fennell explains the 3,000-lb system is packed and shipped in a specially designed container. This equipment resides in the pharmacy or nuclear medicine department for the entire length of the lease. On a weekly basis, source vessels containing the radioactive molybdenum-99 source material are shipped to the user. These sources have a typical useful period of two weeks, after which the source vessel and spent molybdenum are shipped back to NorthStar for recycling.
Fennell says, “The design of the reagent module and its packaging has always been focused on ensuring the end user has a safe, easy-to-use disposable that mitigates the potential for mistakenly connecting a reagent to the wrong port on the RadioGenix system. This packaged module provides that and protects the filled bags during distribution.”
Along with the tri-fold, a programmed RFID tag, tube guide, syringe and scrub caps are kitted, with the entire system double-bagged and placed in a corrugated box for storage in a humidity-controlled environment awaiting shipment to radiopharmacies nationwide.
Once at the pharmacy, the pharmacist removes the outer bag and cleans the inner overbag, which is taken into the cleanroom where it’s opened, with all items in the kit removed for use. The clear tri-fold makes identification of the product easy and also allows the product to stably stand upright. The pharmacist then releases a panel door at the base of the package allowing the tubing and couplers to drop down for easy connection to the instrument in a sequential order.
When the liquid reagent bags have been depleted, the pharmacist disconnects the tubing and disposes of the entire package without ever touching the filled bags. The only point of contact is with tubing that is attached to the bags contained in the lower cavity of the package. Access to the tubing is restricted to a perforated 360-degree tearaway panel door.
“The unique packaging design resulted from NorthStar’s desire to consolidate a number of key components into one module and Barger’s ability to make these concepts a reality,” states Ray Heasty, NorthStar Mechanical Engineer. “The resulting reagent pack is easy to store, install and dispose of after use while performing in contents’ inspections, providing a real benefit for customers using NorthStar’s RadioGenix System. Barger has been terrific to work with from the very start. The Barger team listened to our concerns and the goal we had in mind with this project.”
Mike Motl, Barger’s Senior Product Designer, believes, “Good packaging is like a movie soundtrack. It resides in the background and supports the situation. It’s usually the first barrier in a process to overcome, but making packaging intuitive can take away any unnecessary hurdles. If it’s logically laid out and protective of the medical device inside, it almost becomes an afterthought to the user, while strengthening the perception of the product and brand. With the NorthStar Medical Radioisotopes’ product and package, the customer is fully supported through the design cycle, through concept, prototyping, tooling, specification and product development.”
How have NorthStar customers responded to this new packaging? Fennell says, “Initial response has been favorable. It’s easy to use, requires fewer manipulations, can be readily inspected without opening and serves as a protective overpack for the contents.”
Thermoforming process yields benefits
• Technical advances: Using a Barger-designed and engineered hybrid trim tool, all actions, including trimming, perforating and punching, are performed with one tool in an efficient, single action. The perimeter and hang-hole trim, along with panel door perforations are performed concurrently in two vs three trim planes accomplished through the recession of the hang-hole feature. To ensure the hang-holes match up when assembled, holes are punched out at the same time.
• Tooling design: Allows material to stay in the tool ensuring no scrap or loose materials remain attached to the final package.
• Design advances: The design required an atypically high amount of forming above and below the sheet line to create a multi-level final product. The PETG gauge allows for structural rigidity, with the pyramidal shape and geometry designed for additional strength and package integrity. Draft angles were designed to provide the ability to not only allow the packaging to denest easily without the assistance of stack lugs but to also provide additional rigidity to the package.
• Technology transfer to another industry: Medical devices are commonly stored and shipped in trays using rigid PETG material while tri-fold containers are more commonplace for consumer goods products. Although atypical in the medical device packaging industry, the PETG tri-fold containers design is purposeful and intentional in order to fit the entire systems exact form, fit and function.
• Testing methods for protection: 4.1 ASTM D4169-14, 4.2 PDA TR 27, 4.3 USP<1207>, 4.4 FDA Guidance.
• Economics: Package costs were minimized due to the design and build of a unique, single hybrid tool that enables trimming, perforating and punching in a single operation. Additionally, the number of trim planes were reduced from three to two due to the recession of the hang-hole feature.