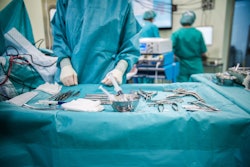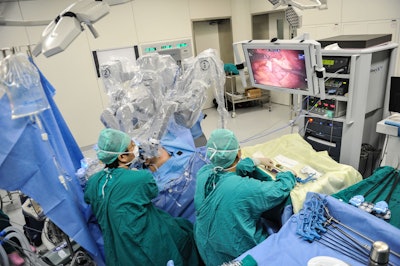
There are a variety of benefits associated with robotic surgery including reduced pain, blood loss, scarring, infection, and recovery time. However, a recent NBC News article says the FDA is now warning against the use of surgical robots for breast and cervical cancer surgery. Apparently there is now limited preliminary evidence that use of the devices may be associated with lower long-term survival if they haven’t been granted marketing authorization by the agency.
If a device hasn’t received marketing authorization from the FDA, it means that the agency hasn’t been able to compare the survival benefits to traditional surgery. The article goes on to discuss the famous da Vinci Surgical System, which has been cleared by the FDA for specific procedures, but not for the prevention or treatment of cancer. Intuitive, the company that manufactures the da Vinci machine notes that there are over 15,000 peer-reviewed articles supporting the safety and effectiveness of robotic-assisted surgery.