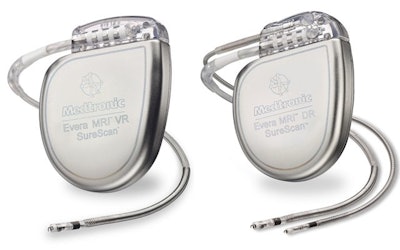
A recent FDA Medical Device Safety report discussed the recall of two Medtronic devices due to a defect in the manufacturing process. A total of nine CRT-Ds and 12 ICD models were affected and included in the Class I recall issued by the FDA on Monday. Class I is the most serious type of recall and was assigned to these devices because the defect may prevent them from delivering life-saving electrical shock therapies.
The defect “causes an out of specification gas mixture inside the device and may prevent the device from delivering the electrical shock needed to pace a patient’s heartbeat or revive a patient in cardiac arrest,” the agency said. “The delay or inability to deliver a shock to a patient in cardiac arrest or pace a patient’s heart whose heartbeat is too slow could result in serious injury and/or death.”





















