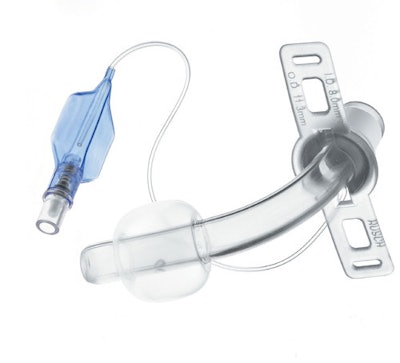
On October 21st, the FDA issued a recall notice for TeleFlex Medical’s Willy Rusch TracheoFlex Tracheostomy Tube Set. The recall is identified as Class I, meaning the use of affected devices could lead to serious injuries or death. The device is a single-use tracheostomy tube used to create safe airway access during a procedure to remove fluid from the trachea and lungs.
Connector components on devices distributed between July 2015 and May 2016 could potentially disconnect from the tracheostomy tube, depriving the patient of proper ventilation during the procedure. Should this happen, immediate care is required to replace the affected device to prevent oxygen deprivation that could lead to brain damage or death.