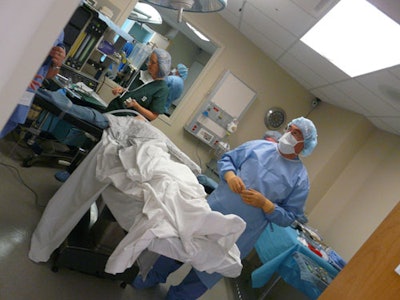
Differences in the operating room and emergency room environments need to be considered in medical device package design. So do multiple factors that are seemingly unrelated, but lead to product waste. Those were two key “takeaways” noted by Dr. Laura Bix, associate professor at Michigan State University's School of Packaging, during MSU's two-day October event described as the nation's first "Healthcare Packaging Immersion Experience" for medical device professionals and health care practitioners.
MSU's press release said, “In an emergency room, precious seconds save lives. They are seconds that cannot be wasted, especially on getting a medical device to work properly or finding out that the packaging on a life-saving device has changed."
Bix tells Healthcare Packaging that MSU's School of Packaging, colleges of College of Human Medicine, Osteopathic Medicine, Nursing and Vet Medicine teamed up with Oliver-Tolas Healthcare Packaging to put together the Oct. 7 and 8 event at MSU's on-campus Learning and Assessment Center (LAC).
About 50 people attended this “pilot” event, Bix says, including medical device manufacturers and suppliers, as well as MSU students and faculty. “Oliver-Tolas has some MSU alumni, so Jane Severin and Teri Meadow at Oliver sat with down with Mary Kay Smith, acting director of the LAC and coordinator of simulation operations, and myself to brainstorm ways that we could put our collective resources together for the benefit of the industry. What developed was a tremendous opportunity with the LAC simulation center to see how package design plays a role in medication errors and sterile presentation. We determined we could look at the way design impacts aseptic presentation, the way healthcare professionals inspect the sterile barrier, and how to address some of the problems and optimize package designs. This event was very focused on medical devices, but we may explore the pharmaceutical side in the future.”
Bix says that MSU and Oliver-Tolas are already planning to host a bigger event October 5-6, 2011, at a MSU site that could accommodate100 people, with nurses and doctors participating to discuss packaging challenges and potential solutions with packaging supplier personnel.
"Our goal is to provide a bridge between the people designing and manufacturing the devices and packaging and the real world," says Bix. "It's important to know how what you as a packaging engineer produce impacts outcomes in the emergency or operating rooms."
Smith points out that having participants experience a simulated surgery and an emergency trauma event can be extremely important. "Packaging engineers and manufacturers are not physicians or technicians in either the emergency or the operating rooms. Simulations provide an opportunity to evaluate how devices work--and don't--in a safe environment. Ultimately, this can lead to better-quality patient care."
Bix says the first day of the event focused on OR simulations where “the environment is more controlled than in an emergency room. There is much more time to prep. The next day in the emergency department (ED) was meant to show participants the difference that context can make with regard to the packages and the labeling. The emergency department involves a much more chaotic, fast-paced approach. We had debriefs with the entire healthcare staff after the simulations and the ED staff said, 'Save us seconds and you save us lives.'”
Asked what she felt were the key takeaways from the two-day event, Bix pointed to the following:
• ER/ED vs OR. “It was clear that the contexts between ED and OR are quite different and that impacts what's crucial to both environments, and what type of packaging features they are able to use. For instance, in the OR, it may be quite easy for them to look at the sterile barrier and determine whether or not the sterile barrier is intact. In ED, they just don't have the time.”
• Waste. “Watching the immersions unfold, what struck me was the amount of waste that happens as a result of packaging, and the potential that it has for the U.S in terms of healthcare dollars spent. One of the nurses was asked, 'How many times do you have an issue with packaging given the number of procedures that you do?' She estimated one in every five procedures had some kind of packaging issue, which is pretty astounding. She was asked what usually happened if there was an issue with presenting the product to the sterile field, and she said 'if it's a low-dollar item I am probably going to pitch it out.'” Bix points out that depending on billing procedures, either the patient or hospital would foot the bill for such waste.
• Sealing-related issues. Bix explains medical device package seals also play a central role in the waste issue. “If your seal is welded shut and it forces them to remove the medical product in a way that doesn't maintain sterility if it flops on the floor or it flops off the sterile field when the seal finally is opened, then you've wasted not only the package, but also the product. The seals could be too strong or they could pop open in transit because they're too weak. I think design is an interesting challenge that we are trying to address with these conferences. Tracking the information and understanding how the package is failing, and what can be done about it presents a rich opportunity that could potentially save costs in the system and minimize a lot of waste.”
-Jim Butschli, Editor, Healthcare Packaging
MSU's press release said, “In an emergency room, precious seconds save lives. They are seconds that cannot be wasted, especially on getting a medical device to work properly or finding out that the packaging on a life-saving device has changed."
Bix tells Healthcare Packaging that MSU's School of Packaging, colleges of College of Human Medicine, Osteopathic Medicine, Nursing and Vet Medicine teamed up with Oliver-Tolas Healthcare Packaging to put together the Oct. 7 and 8 event at MSU's on-campus Learning and Assessment Center (LAC).
About 50 people attended this “pilot” event, Bix says, including medical device manufacturers and suppliers, as well as MSU students and faculty. “Oliver-Tolas has some MSU alumni, so Jane Severin and Teri Meadow at Oliver sat with down with Mary Kay Smith, acting director of the LAC and coordinator of simulation operations, and myself to brainstorm ways that we could put our collective resources together for the benefit of the industry. What developed was a tremendous opportunity with the LAC simulation center to see how package design plays a role in medication errors and sterile presentation. We determined we could look at the way design impacts aseptic presentation, the way healthcare professionals inspect the sterile barrier, and how to address some of the problems and optimize package designs. This event was very focused on medical devices, but we may explore the pharmaceutical side in the future.”
Bix says that MSU and Oliver-Tolas are already planning to host a bigger event October 5-6, 2011, at a MSU site that could accommodate100 people, with nurses and doctors participating to discuss packaging challenges and potential solutions with packaging supplier personnel.
"Our goal is to provide a bridge between the people designing and manufacturing the devices and packaging and the real world," says Bix. "It's important to know how what you as a packaging engineer produce impacts outcomes in the emergency or operating rooms."
Smith points out that having participants experience a simulated surgery and an emergency trauma event can be extremely important. "Packaging engineers and manufacturers are not physicians or technicians in either the emergency or the operating rooms. Simulations provide an opportunity to evaluate how devices work--and don't--in a safe environment. Ultimately, this can lead to better-quality patient care."
Bix says the first day of the event focused on OR simulations where “the environment is more controlled than in an emergency room. There is much more time to prep. The next day in the emergency department (ED) was meant to show participants the difference that context can make with regard to the packages and the labeling. The emergency department involves a much more chaotic, fast-paced approach. We had debriefs with the entire healthcare staff after the simulations and the ED staff said, 'Save us seconds and you save us lives.'”
Asked what she felt were the key takeaways from the two-day event, Bix pointed to the following:
• ER/ED vs OR. “It was clear that the contexts between ED and OR are quite different and that impacts what's crucial to both environments, and what type of packaging features they are able to use. For instance, in the OR, it may be quite easy for them to look at the sterile barrier and determine whether or not the sterile barrier is intact. In ED, they just don't have the time.”
• Waste. “Watching the immersions unfold, what struck me was the amount of waste that happens as a result of packaging, and the potential that it has for the U.S in terms of healthcare dollars spent. One of the nurses was asked, 'How many times do you have an issue with packaging given the number of procedures that you do?' She estimated one in every five procedures had some kind of packaging issue, which is pretty astounding. She was asked what usually happened if there was an issue with presenting the product to the sterile field, and she said 'if it's a low-dollar item I am probably going to pitch it out.'” Bix points out that depending on billing procedures, either the patient or hospital would foot the bill for such waste.
• Sealing-related issues. Bix explains medical device package seals also play a central role in the waste issue. “If your seal is welded shut and it forces them to remove the medical product in a way that doesn't maintain sterility if it flops on the floor or it flops off the sterile field when the seal finally is opened, then you've wasted not only the package, but also the product. The seals could be too strong or they could pop open in transit because they're too weak. I think design is an interesting challenge that we are trying to address with these conferences. Tracking the information and understanding how the package is failing, and what can be done about it presents a rich opportunity that could potentially save costs in the system and minimize a lot of waste.”
-Jim Butschli, Editor, Healthcare Packaging