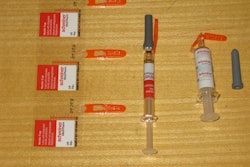
Aptar Pharma’s Pro-Ject® is an auto-injector that has been designed and developed with input from patients and healthcare professionals to provide optimal patient convenience and compliance.
It is now generally accepted that human factor engineering is an integral part of developing any new drug delivery device. Human factor studies address many different ‘human elements,’ such as perceptual, cognitive, ergonomic, emotional, social, and even cultural. The outcome of human factor studies should provide a clear understanding of how to avoid errors, identify safety risks, and improve patient education/training and competence as well as patient compliance.
In addition, government authorities in the United States and Europe now offer regulatory guidance that outlines how to integrate human factor studies into drug delivery devices during their development to optimize their design and gain successful regulatory approval.
Aptar Pharma applied human factor guidelines to the Pro-Ject® development cycle. During the initial Pro-Ject® concept phase, Aptar Pharma carried out several investigations and market studies with patients and healthcare professionals. The main objective of these studies was to better understand users’ and healthcare professionals’ experiences, expectations, and preferences for auto-injectors.
Once a prototype of Pro-Ject® had been developed, additional user acceptance studies and investigations were performed to deepen the understanding of how rheumatoid arthritis patients interacted with Pro-Ject®. Findings from these studies were discussed during Pharmapack Europe, February 12-13, 2014.
During the studies, patients provided invaluable insights on these topics:
- First impressions
- Key preferences and suggestions for improvement
- Perception of how the device handles, including ease of use, intuitiveness, and user feedback
- General interest in using the device
Results demonstrated that both training nurses and rheumatoid arthritis patients confirmed the benefits of Pro-Ject® in terms of ease of use, ergonomics, and design. Pro-Ject® integrates all of the user-preferred features that were identified during user studies. These features include:
- Push-on-skin activation. Some users with diseases such as rheumatoid arthritis cannot use their thumbs to push a button to activate the device.
- Slow injection. Auto-injectors that deliver drugs too fast are perceived as being painful.
- Hidden needle. Some patients become anxious when they see needles, and anxiety can make injections more painful.
- Large and highly visible indicator window. Coupled with this, users prefer an end-of-injection color indicator.
- Audible feedback. Patients appreciate hearing audible clicks at the beginning and the end of the injection process.
Self-injection and the auto-injector market
Current estimates value the global market for injectable drugs at U.S. $240 billion, giving injectables a 28% share of the overall drug market worldwide. The growth rate for the injectable drug market was 4% in 2012. The main driver for this growth is the category of drugs known as biologics, which are large molecules manufactured by biotechnology processes. Biologics need to be administered by injection, given their size and profile.
Auto-injectors are used in the treatment of chronic diseases such as rheumatoid arthritis, lupus, psoriasis, spondilarthritis, multiple sclerosis, hepatitis C, anemia, and for emergency/crisis treatments, such as anaphylactic shock and migraine attacks by self-injection in a non-medical environment. The use of auto-injectors by properly trained patients does not require the presence of healthcare professionals.