Cryoport, Inc., a provider of advanced cryogenic logistics products for the life sciences industry, is enhancing its cryogenic logistics support of its Sanaria partnership, which includes Sanaria's production of malaria vaccine as well as its research and development efforts.
Rockville, MD-based biotechnology company Sanaria's website says its “primary mission is to develop and commercialize whole-parasite sporozoite vaccines that confer high-level, long-lasting protection against Plasmodium falciparum, the malaria parasite responsible for more than 95% of malaria associated severe illness and death worldwide and the malaria parasite for which there is the most significant drug resistance.”
Plasmodium falciparum is thought to be responsible for more childhood mortality than any other single infectious agent. Due to the nature of the disease, Sanaria's vaccine developments are focused on Africans, particularly children, who are considered the most at risk. The company's flagship product, Sanaria(®) PfSPZ Vaccine was reported in 2013 to provide 100% protective efficacy in a clinical trial at the U.S. National Institutes of Health. Sanaria also makes available to the research community a range of parasitological and entomological reagents and assay services.
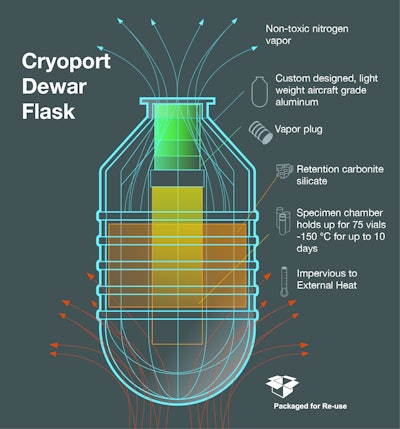
Distribution of vaccines into the developing world continues to be a significant challenge to disease prevention efforts. Sanaria and Cryoport are actively working toward development of a robust distribution network through use of a liquid nitrogen vapor phase (LNVP) cold chain, which both believe is critical to the success of vaccine/ biologics distribution throughout the African subcontinent, and provides significant advantages over current biologics delivery systems.
Dr. Stephen L. Hoffman, Sanaria's Chief Executive and Scientific Officer says, "Our mission is to develop a vaccine that will combat a malaria parasite that is the most drug-resistant and causes more than 98% of malaria related deaths worldwide.With Cryoport as our logistics partner, we can trust that their validated cold chain logistics solutions will provide a reliable and effective distribution strategy to bring to market an effective preventative vaccine for this deadly disease. We also rely on Cryoport for delivery of our family of products that are intended to advance and promote high quality research for new vaccines and drugs."
Jerrell Shelton, Cryoport's CEO, states, "We are proud to be able to support Sanaria's groundbreaking work to eliminate malaria. Our global reach is fully supportive of Sanaria's multi-center and multi-country clinical trials, and will be able to support delivery of the vaccine to the hundreds of millions of individuals who need it.”