Dr. Pfleger Arzneimittel GmbH is a Germany-based healthcare company that has operated for nearly 80 years with the motto that people are what counts.
They manufacture, develop, and distribute more than 60 medicinal products and medical devices in the fields of urology, dermatology, and gynecology, as well as home care medication for pain, hoarseness, and hair health.
“The work at Dr. Pfleger is always focused on the well-being of people. Because, for the company, it is people who count—yesterday, today, and in the future,” the company reports. “This focus on people applies down to the tiniest detail. Because Dr. Pfleger is well aware: It is not only the company’s own medicinal products themselves but also related topics, such as the packaging solutions used, that are an important contribution to future-oriented and sustainable actions. For the environment and thus for people.”
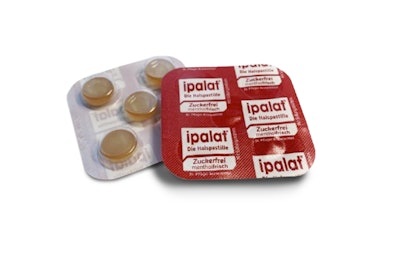
In March 2021, Dr. Pfleger approached ETIMEX Primary Packaging GmbH to develop a fully recyclable blister for their well-established ipalat throat pastilles as a sample. In addition to being offered in an aluminum can format, this initiative aimed to introduce sustainability to customers by providing product samples in a recyclable packaging variant using the PURELAY polypropylene (PP) monoblister.  The PURELAY PP monoblister from Etimex.
The PURELAY PP monoblister from Etimex.
From test run to product samples
Once they identified their packaging goal, they commenced development work. Dr. Pfleger, in collaboration with ETIMEX, embarked on a rigorous testing phase utilizing the new material. Dr. Pfleger received several small rolls of the mono PP to conduct numerous test runs on their own machinery. Having ETIMEX developers on-site was helpful for efficient testing—they were able to assist directly for machine conversion.
After successful testing, they produced an initial monoblister to check the new design for haptics and ease of push-through. The pharmaceutical industry faces unique challenges on the way to a new package: sample preparation times are typically long since specifications must be met as precisely as possible.
Sample in the new monoblister
Despite these hurdles, after two years of development, they successfully validated the PURELAY blister. The monoblister is now available to consumers, allowing them the opportunity to sample products through individually removable pastilles.
“This sustainable solution gives us the opportunity to have ipalat throat pastilles tested by our future customers. In this way, they can convince themselves of our product before they buy it,” says Corinna Spies, brand manager at Dr. Pfleger Arzneimittel.
As Dr. Pfleger highlights, the world needs future-oriented companies across all sectors, and with the long development and testing times that pharmaceutical companies face, there’s no time like the present to begin. Dr. Pfleger Arzneimittel's commitment to people-centric innovation prioritizes forging new paths that positively impact both individuals and the environment.






















