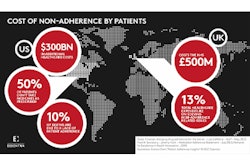
Between 20 and 50 percent of patients fail to adhere to their medication regimens. The issue costs $290 billion a year in the U.S., making patient compliance a major concern for pharmaceutical companies. However, a recent Business Wire article noted the development of a new patch aimed at helping patients comply. E Ink and LTS have created a “Smart Patch” prototype that delivers medication and features a display with important information.
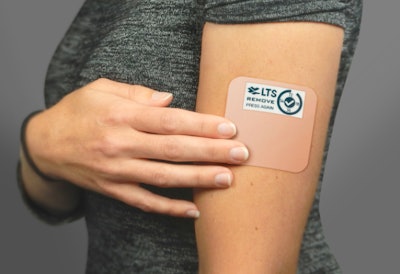
The Smart Patch features a 2” electronic paper display powered by a bistable circuit so there’s no need to recharge the battery. It also contains a switch, pressure sensor, and displays a message indicating the patch is properly applied to the skin, a countdown until the next dose, and a reminder to remove the patch and replace it. The Smart Patch will begin targeting treatment of chronic diseases before moving on to other applications.