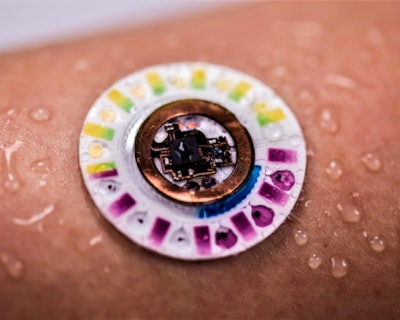
Consumers and medical professionals are no stranger to wearable patches, employing them to monitor exercise regimens, track vital signs, blood pressure, glucose levels and other health information.
As long ago as the 1960s, relates Packaging Hall of Fame inductee Ben Miyares, we’ve had transdermal patches, one of which he recalls, functioned like a “contact lens,” releasing eye medication in a controlled fashion.
An intriguing new patch type is currently in development at the intersection of advanced materials and device innovation that employs Epifluidics (epidermal + microfluidics) technology. It is described in a Jan. 18 New York Times article as “A new device—wearable, wireless and battery-free—[that] improves the ability to monitor and diagnose health problems by analyzing the sweat on your skin.
“The new device has minuscule holes at its base into which sweat naturally flows. From there, a complex network of valves and microchannels, each roughly the width of a human hair, route the sweat into tiny reservoirs. Each reservoir contains a sensor that reacts with a chemical in the sweat, such as glucose or lactate,” the story explains.
The “key architect of the device,” said the NYT story: Prof. John Rogers, Biomedical Engineer at Northwestern University. Rogers told the NYT, “It fits into a broader trend that you’re seeing in medicine, which is personalized, tailored approaches to treatment and delivery of care.”
To learn more about the “sweat patch,” we followed up with Rogers’ co-author of the research article, Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat, describing the technology, Roozbeh Ghaffari, PhD. Ghaffari is CEO/Co-Founder of Epicore Biosystems, a spin-off of the Northwestern lab, tasked with pursuing commercialization of the sweat microfluidics technology.
Healthcare Packaging (HCP): How will this patch be used?
Ghaffari: Our research paper showcased “Electronics-Enabled Epifluidics” devices, highlighting the integration of electrochemical sensors onboard epifluidic devices. Epifluidic devices have important medical applications. We anticipate this technology being deployed in clinical settings and at home to monitor patients remotely. On the commercialization front, we have initially focused on consumer wellness and beauty and sports applications, with the goal to expand into more regulated applications with medical claims in the future.
HCP: Is the patch is being evaluated for its potential to identify or address several different diseases/conditions?
Yes, we have active studies with clinical collaborators in progress where we are testing epifluidic devices in stroke rehabilitation, cystic fibrosis screening and kidney disease screening, to name a few.
HCP: When might these patches be FDA-approved and ready for use commercially?
We are in the process of running multiple feasibility studies and are focused on launching consumer health/wellness products currently with industrial and military partners. FDA approval and commercial launch are ~24 months out. Based on our current capabilities there are interesting applications in cystic fibrosis, diabetes, hyperhidrosis and stroke where these devices could provide insight outside of controlled clinical environments, which does not exist today.
HCP: How is a patch “programmed” to screen for a particular disease?
Epifluidic patches can be prepared with different biochemical assays and designed to support sweat volumes depending on the use case. For pre-diabetes screening, we measure glucose concentrations in sweat. For cystic fibrosis, we measure chloride concentrations in sweat. The overall volume of sweat captured depends on the size, dimensions and geometry of a given epifluidics patch design.
HCP: What materials make up the patches?
Epifluidic patches tend to be a few hundred micrometers in thickness and extremely soft on the skin and to the touch. The microchannel dimensions are designed to support sweat flow directly from skin pores without requiring any form of actuators, battery power or valves. The device layers are typically molded or could be assembled in layer form with multiple layers of material in a stack. The materials do not need to be assembled in a cleanroom, though they could be if that is a requirement.
HCP: How do you envision such patches meeting personalized medicine treatment and delivery of care issues?
These patches could be deployed in the clinic or at home. They provide information about your biochemical state outside of the hospital and do so non-invasively. From hydration and stress to disease screening, there are several applications where this class of device can offer information about the state of health, performance and recovery, and how they may change over time. This biochemical/metabolic insight is overlooked by most wearable devices on the market today.
HCP: Would a patch be produced for a single person, or to detect a specific condition?
These are one-time use patches. In some instances, the electronics module can be reused. The sweat microfluidic substrate is intended to be disposable for single or multi-use applications. Each epifluidic patch can be tuned for a specific disease target with slight variations in design and with specific bioassays targeting different diseases.
HCP: Would these patches likely be subject to any unusual conditions in use?
Epifluidic devices are designed to be waterproof for use in the shower and during swim/triathlon exercises. The device is fairly resilient to varying environmental conditions. In some cases, the bioassays that are inside the patch could be sensitive to varying temperatures and should be kept refrigerated for optimal performance.
HCP: What properties would such patches require of their packaging?
Packaging is a crucial part of deployment for epifluidic devices. We are working on manufacturing these devices and developing application-specific packaging. It is a bit early to determine whether new classes of packaging will result.
HCP: Are there plans for these patches to one day deliver medication to treat specific diseases? How would those differ from the current patches that gather information through the patient’s sweat?
Combining therapy with the diagnostic/monitoring capabilities is something we have considered and find extremely interesting. We are still in the early stages of developing our suite of sweat dynamics/analytics capabilities and will likely explore ways to combine this capability set with transdermal drug delivery and other forms of therapeutic stimulation in the future.
SIDEBAR:
Mass Customization and Epifluidic Devices
PMMI’s “Vision 2025 Report” identifies the term mass customization as “the consensus ‘go to’ phrase describing the challenges faced by Vision 2025 participants in understanding consumer and customer demands and the operational tactics required for fulfilling them.” The report cites Investopedia’s description of mass customization “as a marketing and manufacturing technique that combines the flexibility and personalization of custom-made products with the low unit costs associated with mass production.”
Roozbeh Ghaffari, PhD, sees the devices fitting into the mass customization approach. He says, “We have found useful ways to reduce cost and with minor changes in design across different applications. We will learn a lot more over the coming years as epifluidic products enter the marketplace. One of the key challenges lies in how we automate certain capabilities required to align with mass production. There is some upfront investment, effort and cost associated with this automation. The projected volumes and single-use nature of epifluidic devices are driving us in this direction.”
Download PMMI Vision 2025 Report here.