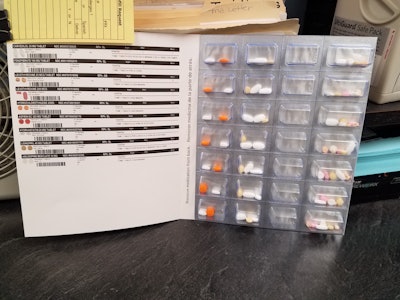
When patients testify that packaging may well save their lives, you know you’re on the right track.
That’s precisely how the Holyoke Health Center (HHC) sees its ongoing switch from traditional medication vials to SureMed® multi-medicine blister cards from Omnicell Inc.
HHC is a federally qualified, non-profit community health center in Holyoke, MA, with a second site in nearby Chicopee, with dental clinics at each location in addition to facilities as well in Westfield.
Challenges in healthcare
Lori Lewicki, RPh, and HHC Pharmacy Director, explains that the facility serves a mix of patients with challenges that range from high health illiteracy, language issues (many patients do not speak English), as well as physical challenges due to age or chronic disease. Many patients take double-digit numbers of medications, often more than once a day, further complicating their ability to comply with critical medication regimens.
And while the concept of multiple medications in calendared blister packs may be initially confusing, the ultimate result is better patient outcomes as well as financial benefits for HHC and the state’s overall healthcare system.
Says Lewicki, “Our customers absolutely love the Med Box/SureMed packaging, especially if it's an elderly person that couldn't handle taking numerous different medications from different vials or bottles. We have a primarily Hispanic population, many of whom are diabetic. When our facility had an expansion a couple of years ago, we had two patients provide testimonials about our pharmacy and how the new packaging was helping them keep their medications straight. One man said he would have died by now without it. He said, ‘There's no way that I could manage my healthcare and open all these bottles and take all of my pills.
“We also had a woman tell us that she could never remember whether or not she took her meds [with the traditional vial]. She is very appreciative,” Lewicki continues. “With the Med Box our patients do not have to call in for refills. Once they sign onto our Med Box program, the packaging helps us to track when they need refills and keep them in adherence.”
Community Health Workers call three days in advance of the Med box due date to see if there have been any changes and to get the patient’s permission to refill the medications. If there have been any changes, a pharmacist intervenes to update the medlist and the box.
HHC provides adult and pediatric primary medical and dental services, with both community and clinical pharmacy services, providing everything from health insurance outreach, access, and enrollment services to educational and support services for weight and chronic disease self-management, HIV/AIDS counseling, testing and substance abuse services and Hep C counseling and treatment.
With more than 300 employees that include medical and dental providers, many of whom are bilingual, as well as case managers, outreach workers, and financial counselors, HHC serves nearly 23,000 patients annually, or nearly 50,000 medical visits and 43,000 dental visits each year.
Lewicki explains that HHC’s in-house pharmacy is divided into three parts:
• A “community portion” that serves much like a retail pharmacy, but with a 340B program whereby it is allowed by government to purchase medications at up to 50% to 60% off regular prices.
• A Medication Therapy Management (MTM), or Integrated Medication Management (IMM) that allows direct communication between a pharmacist and a provider, allowing a patient to make an appointment with an HHC pharmacist.
• A compliance packaging program in which the Omnicell packaging and robotic pharmacy automation systems are used to produce personalized medicine blister packs for patients. Typically, patients in the MTM program are often referred into the Omnicell program, which Lewicki refers to as MedBox, or Suremed cards.
Read Part II of this story.
Read Part III of this story.