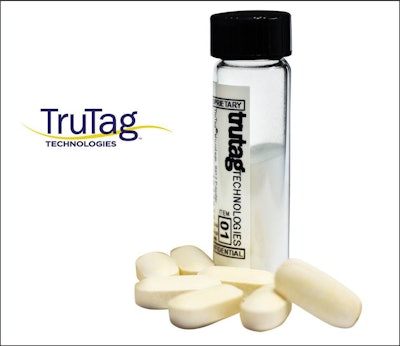
TruTag Technologies, Inc.’s portable optical reader can authenticate solid oral-dose medicines marked with the TruTag® microtag.
TruTag's "covert, edible bar codes" provide drug makers with a product security and tracking solution at the dosage level, rather than on packaging, so that manufacturers can authenticate medicine directly on a pill with the ability to confirm the product strength, batch number, site of manufacture, and expiration date, among other product information.
In late 2011, the U.S. Food and Drug Administration issued final guidance to the drug industry for the use of physical-chemical identifiers (PCIDs) as on dose authentication measures like TruTag's solution in drug products. This FDA guidance provides a framework for pharmaceutical manufacturers to begin implementing PCIDs in solid oral-dosage form drug products.
TruTag's high-purity silica microtags are inert and can be associated with a variety of product information, similar to a traditional bar code. Further, silica (silicon dioxide) has long been used as an excipient in food and medicine, and is well-suited for the PCID Guidance because it has been affirmed as "generally recognized as safe," or GRAS, by the FDA.
TruTag's novel drug authentication technology won the 2010 North American Pharmaceutical and Biotechnology Innovation of the Year award presented by Frost & Sullivan.






















