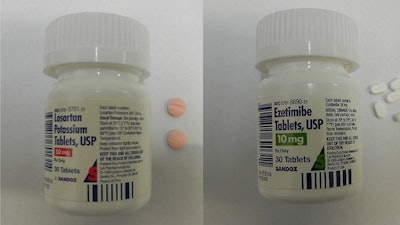
Recalled Drugs / Image: CPSC
A recent Spectrum News article discussed yet another blood pressure medication recall. This time, it’s Sandoz that is recalling more than 600,000 units of two different medications because they weren’t packaged in childproof containers, as required by the Poison Prevention Packaging Act according to the U.S. Consumer Product Safety Commission. The drugs in question are Ezetimibe and Losarten Potassium, and they were sold nationwide in clinics and pharmacies from July 2018 through August 2019.






















