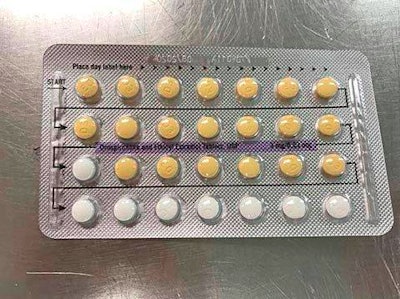
Birth Control Blister Pack / Image: TribLive
A recent prescription recall could hold consequences nine months later. According to a recent Trib Live article, a Florida-based pharmaceutical company issued a recall on birth control pills due to packaging errors. Apotex Corp.’s voluntary recall affects four lots of Ethinyl Estradiol USP pills that may contain defective blisters with the wrong arrangement of pills or empty blisters. The mix-up could lead to unintended pregnancy if the patient takes a placebo instead of an active tablet.