On Dec. 3, FDA’s Consumer Healthcare Information announced that women and their health care providers can look forward to getting more useful and up-to-date information over the next few years about the effects of medicines during pregnancy and breastfeeding.
The improvements will replace the current, decades-old labeling system and implement new requirements by FDA regarding labeling information for prescription drugs and biological products. The changes will include major revisions in the subsections about pregnancy and breastfeeding.
“FDA wants pregnant and breastfeeding women and their health care providers to benefit from the most useful and latest information about their prescription medicines,” says Sandra Kweder, M.D., Deputy Director of FDA’s Office of New Drugs. She says pregnant women and health care providers need this information because:
• Women take an average of three to five medications during pregnancy
• Many pregnant women have chronic conditions—such as asthma, high blood pressure, depression, and diabetes—that require them to continue taking medications they were on before pregnancy
• New health problems may begin or old ones may get worse during pregnancy, requiring treatment
• A woman’s body changes throughout her pregnancy, which may affect the medication dose she needs
The revised labeling will include more information on whether medication gets into breast milk, and—based on how much of it is present—how it can possibly affect the infant.
In addition to the revisions to the subsections about pregnancy and breastfeeding, the labeling will also include a subsection called “Females and Males of Reproductive Potential.” This subsection will provide a consistent location for relevant information about pregnancy testing, birth control and a medication’s effect on fertility.
“For medications that may cause infertility or present risks in pregnancy, the revised labeling will include information to be considered when deciding such issues as birth control or planning a pregnancy,” Kweder says.
Labeling background
Until now, FDA categorized the risks of taking a drug or biological product during pregnancy under a five-letter system (A, B, C, D, and X) based on what was known about that product. But comments received by FDA showed that the letter system was often confusing because it was overly simplistic, and did not reflect the available information. This system could lead to false assumptions about medications based on their category.
“The revised labeling will change that,” says Kweder. “Now doctors will have up-to-date and well-organized information on pregnancy and lactation. They will be in a better position to help their patients make critical decisions.”
Revised labeling
The revised labeling will replace the old five-letter system with more helpful information about a medication’s risks to the expectant mother, the developing fetus, and the breastfed infant.
In addition, the labeling will include contact information for pregnancy exposure registries that collect and maintain data on the effects of medications used by pregnant women. Pregnant women are encouraged to enroll in these studies if they are taking drugs or biological products for which there is a registry.
What’s next
Companies will have to remove the pregnancy letter categories from the labeling for all prescription drugs and biological products and, for many of them, revise the labeling with updated information. This is a large undertaking that will take several years.
Deciding which drug to take is a complex and individualized decision that should be discussed with your doctor, but the new FDA rule helps remove some of its uncertainties.
“The greatest benefit to patients is that these changes will result in better-informed prescribing based on the latest scientific information for thousands of medical products,” notes Kweder. “Our goal is to empower health professionals and patients so that women can have confidence in treatment decisions for themselves and their families.”
“For medications that may cause infertility or present risks in pregnancy, the revised labeling will include information to be considered when deciding such issues as birth control or planning a pregnancy,” Kweder says.
Women and their health care providers can look forward to getting more useful and up-to-date information over the next few years about the effects of medicines during pregnancy and breastfeeding.
The improvements will replace the current, decades-old labeling system and implement new requirements by FDA regarding labeling information for prescription drugs and biological products. The changes will include major revisions in the subsections about pregnancy and breastfeeding.
Pregnancy and lactation labeling final rule
FDA published the “Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling,” referred to as the “Pregnancy and Lactation Labeling Rule” (PLLR or final rule).
The PLLR requires changes to the content and format for information presented in prescription drug labeling in the Physician Labeling Rule (PLR) format to assist health care providers in assessing benefit versus risk and in subsequent counseling of pregnant women and nursing mothers who need to take medication, thus allowing them to make informed and educated decisions for themselves and their children. The PLLR removes pregnancy letter categories – A, B, C, D and X. The PLLR also requires the label to be updated when information becomes outdated.
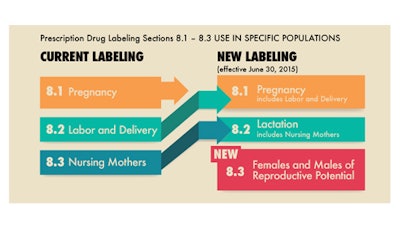
Below is a comparison of the current prescription drug labeling with the new PLLR labeling requirements (also, see attached graphic).
The Pregnancy subsection (8.1) includes information for a pregnancy exposure registry
for the drug when one is available. Pregnancy exposure registries collect and maintain data on the effects of approved drugs that are prescribed to and used by pregnant women. Information about the existence of any pregnancy registries in drug labeling has been recommended but not required until now. Information in the Pregnancy sub-section includes a Risk Summary, Clinical considerations, and Data. Information formerly found in the “Labor and delivery” subsection is now included in the “Pregnancy” subsection.
The Nursing mothers subsection was renamed, the Lactation subsection (8.2), and provides information about using the drug while breastfeeding, such as the amount of drug in breast milk and potential effects on the breastfed infant.
The Females and Males of Reproductive Potential subsection (8.3), new to the labeling, includes information, when necessary, about the need for pregnancy testing, contraception recommendations, and information about infertility as it relates to the drug.
The labeling changes go into effect on June 30, 2015. Prescription drugs and biologic products approved after June 30, 2015, will use the new format immediately, while labeling for prescription drugs approved on or after June 30, 2001, will be phased in gradually.
Labeling for over-the-counter (OTC) medicines will not change; OTC drug products are not affected by the final rule.
Concurrently with publishing the PLLR, FDA also issued draft guidance for industry to assist drug manufacturers in complying with the new labeling content and format requirements.





















