Soon, labels for many medical devices of the higher-risk variety will need to contain "unique device identifiers" (UDIs) to help aid with corrections and recalls and to help thwart counterfeiters.
The U.S. Food and Drug Administration issued a proposed regulation to set up the UDI system in early July, and the public has a chance to comment on the proposal for 120 days, until Nov. 7 of this year.
This is a key time within this long-term process: Though the proposal has been a long time coming, and the final rule won't be fully phased in for years, industry right now has a limited window of opportunity to give FDA comments on this important proposal, which was originally part of FDA's implementation of a Congressional mandate from 1997, when Congress included a requirement for such identifiers in amendments to the Federal Food, Drug and Cosmetic Act.
Of course, in 1997, Congress did not tell FDA it should take this many years to come up with its proposal.Still, FDA's work has encompassed exploration of a variety of approaches, pilot programs to try out identifier systems, and public workshops.
Under the proposed system, labels of most medical devices and device packages would need to feature the UDI in both plain text format and readable tech form (automatic identification and data capture, "AIDC").
Some devices could place the UDI elsewhere, some would have to place it on the device itself, and others are exempt altogether from the requirement, including "devices sold over-the-counter and low- risk devices," says FDA. So, simple devices you can buy on your own won't need it (they commonly have UPC codes on their labels, which serve a lot of the same purposes of a UDI). There would also be a public database of the UDIs and their counterpart devices.
The UDI itself will contain the device identifier (version or model of the device, and identifier for the labeler), and product identifier (identification for the specific device including lot or batch numbering, serial number, expiration data or date of manufacture).
The UDI system is designed to serve many purposes at once, including at least these, says FDA:
1. Reduce medical errors.
2. Simplify the integration of device use information into data systems.
3. Provide for more rapid identification of medical devices with adverse events.
4. Provide for more rapid development of solutions to reported problems.
5. Provide for more rapid, more efficient resolution of device recalls.
6. Provide better-focused and more effective FDA safety communication.
7. Provide an easily accessible source of definitive device Identification Information.
FDA says it received input from a range of interested parties, and designed the program to build on "current standards and systems already in use by some companies" to "minimize industry costs and expedite implementation."
Speaking of input, the next few months represent your opportunity to give FDA your comments on the shape of the rule, but the timetable for implementation won't speed up after that.FDA says the final rule, once it appears after public comments are gathered and reviewed, would be phased in over the course of seven years, applying to higher-risk devices first, to minimize the burdens on the regulated businesses and to make for smoother transition.
--By Eric F. Greenberg, Attorney-At-Law
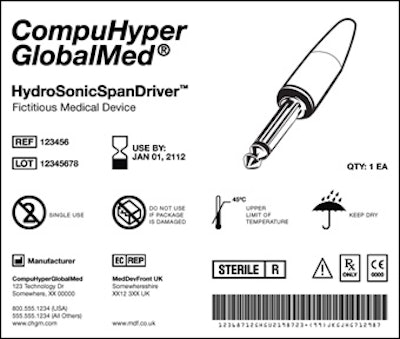
The photo here, provided by the U.S. Food and Drug Administration, shows an example of what a universal device identifier (UDI) would look like on a medical device label. The label contains information about the product name, its expiration date, reference and lot numbers, manufacturer information, bar code, details about the item, and an illustration of the item.
The U.S. Food and Drug Administration issued a proposed regulation to set up the UDI system in early July, and the public has a chance to comment on the proposal for 120 days, until Nov. 7 of this year.
This is a key time within this long-term process: Though the proposal has been a long time coming, and the final rule won't be fully phased in for years, industry right now has a limited window of opportunity to give FDA comments on this important proposal, which was originally part of FDA's implementation of a Congressional mandate from 1997, when Congress included a requirement for such identifiers in amendments to the Federal Food, Drug and Cosmetic Act.
Of course, in 1997, Congress did not tell FDA it should take this many years to come up with its proposal.Still, FDA's work has encompassed exploration of a variety of approaches, pilot programs to try out identifier systems, and public workshops.
Under the proposed system, labels of most medical devices and device packages would need to feature the UDI in both plain text format and readable tech form (automatic identification and data capture, "AIDC").
Some devices could place the UDI elsewhere, some would have to place it on the device itself, and others are exempt altogether from the requirement, including "devices sold over-the-counter and low- risk devices," says FDA. So, simple devices you can buy on your own won't need it (they commonly have UPC codes on their labels, which serve a lot of the same purposes of a UDI). There would also be a public database of the UDIs and their counterpart devices.
The UDI itself will contain the device identifier (version or model of the device, and identifier for the labeler), and product identifier (identification for the specific device including lot or batch numbering, serial number, expiration data or date of manufacture).
The UDI system is designed to serve many purposes at once, including at least these, says FDA:
1. Reduce medical errors.
2. Simplify the integration of device use information into data systems.
3. Provide for more rapid identification of medical devices with adverse events.
4. Provide for more rapid development of solutions to reported problems.
5. Provide for more rapid, more efficient resolution of device recalls.
6. Provide better-focused and more effective FDA safety communication.
7. Provide an easily accessible source of definitive device Identification Information.
FDA says it received input from a range of interested parties, and designed the program to build on "current standards and systems already in use by some companies" to "minimize industry costs and expedite implementation."
Speaking of input, the next few months represent your opportunity to give FDA your comments on the shape of the rule, but the timetable for implementation won't speed up after that.FDA says the final rule, once it appears after public comments are gathered and reviewed, would be phased in over the course of seven years, applying to higher-risk devices first, to minimize the burdens on the regulated businesses and to make for smoother transition.
--By Eric F. Greenberg, Attorney-At-Law
The photo here, provided by the U.S. Food and Drug Administration, shows an example of what a universal device identifier (UDI) would look like on a medical device label. The label contains information about the product name, its expiration date, reference and lot numbers, manufacturer information, bar code, details about the item, and an illustration of the item.