An August 16th FDA article noted 36% of U.S. adults are considered obese, linking them to health issues like heart disease, diabetes, and high blood pressure. There are four types FDA-approved medical devices for high BMI patients who need assistance beyond diet and exercise. They are:
Gastric Bands
These surgically implanted bands wrap around the stomach to decrease its size, helping patients eat less.
Electrical Stimulation System
This implanted device sits in the abdomen and blocks nerve activity between the brain and stomach. It consists of nerve electrodes, wire leads, and a rechargeable electrical pulse generator that delivers electrical signals to electrodes. External controllers allow physicians to adjust settings and patients to charge the device.
Gastric Balloons
This device consists of either one or two balloons that essentially take up space in a patient’s stomach for six months. They’re inserted using an endoscope and then filled with saline and sealed.
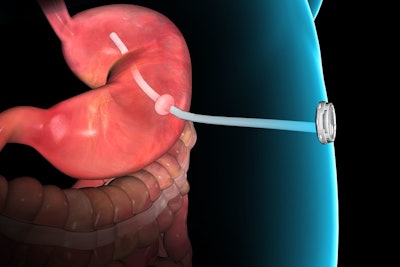
Gastric Emptying System
This recently approved device is basically a tube leading from the stomach to the exterior of the abdomen. Patients are able to empty contents of their stomach after eating, but before the body has a chance to digest.





















