Rockwell Automation released its Rockwell Software PharmaSuite v5.0 system for pharmaceutical and biotech production, setting new standards for manufacturing execution system (MES) performance in automation, risk management, cost reduction, and regulatory compliance. The new version delivers a single MES solution with the agility to integrate and streamline production not only across multiple production areas and product lines, but also from line to site level.
With improved automation-layer integration, equipment modeling and nonorder work-flow management, the PharmaSuite v5.0 system helps manufacturers deploy systems more rapidly with greater production flexibility.
The company said it was working to set new standards in performance, usability, and deployment time with every new PharmaSuite release. Version 5.0 provides a core solution poised to compress the time from deployment to return on investment regardless of the application.
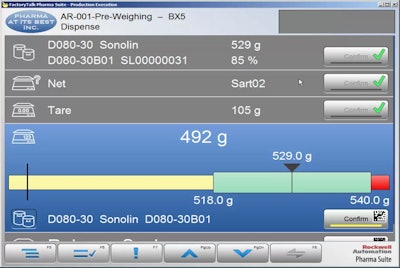
The PharmaSuite v5.0 system offers improved integration with production equipment—including premier integration to the Logix control platform—and automated batch processing using the PharmaSuite Recipe Designer. The system automatically collects data directly from production equipment, provides automation set points, monitors automation events, and integrates process information into the electronic batch record to reduce the risk for human error that can result from manual data collection.
The PharmaSuite Recipe Designer now supports a greater number of production processes. Its enhanced information-flow design enables access to both definition and run-time information across the entire recipe to give users added insight into real-time production information. The new data types within the expression editor enable users to deploy more sophisticated conditions, rules, and calculations so they can more easily customize functionality to meet their needs.
The PharmaSuite v5.0 system uses a new equipment modeler to define the underlying model and optimize integration across the range of manufacturing types, including biological, secondary, active, discrete assembly, weigh and dispense, and packaging. This allows users to define the automation interface, maintain equipment descriptions and specifications, and use the new equipment modeler as the foundation for GMP-compliance tracking and automation-integration scenarios. The user interface provides quick access to production information and is optimized to maintain and analyze massive amounts of equipment-related data.
The PharmaSuite v5.0 system redefines assembling and supporting nonorder-related workflows that traditionally require custom programming by a systems integrator. Using S88-recipe design principles, the PharmaSuite v5.0 system enables the on-site end user or recipe author to create new work flows on their own, in just minutes, by re-using existing building blocks. By creating and managing these back-end tasks on their own, manufacturers can reduce overall production costs to help accelerate the return on investment.






















