This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
During MD&M West on February 7-9 at Booth #1693 in the Anaheim Convention Center in Anaheim, Calif., Stäubli Robotics will demonstrate specialized cleanroom robots used in medical device and pharmaceutical manufacturing, as well as robot-assisted surgery.
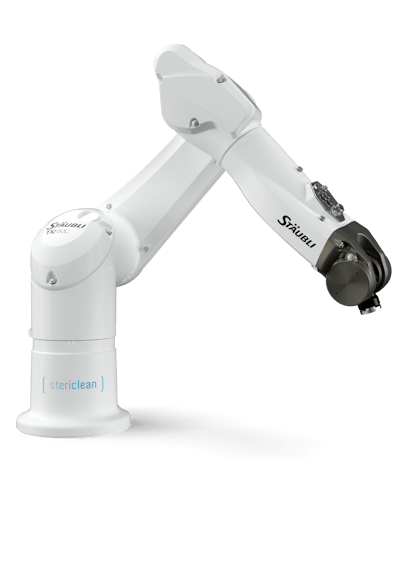
Stäubli’s Stericlean robots are designed for life science and operate safely within Grade A, B, and C environments. They have a fully enclosed structure with special seals to minimize airborne particles, a smooth surface with no retention areas, and a special high-resistance coating. The robots are also highly flexible to adapt to different drug delivery formats and carry out complex tasks in fill-finish, labeling, QA testing, and inspection.
Extending Stäubli’s robots for aseptic applications is the Stericlean+ line, launched in 2022. These robots feature a new fully hygienic design and coating for higher performance in sensitive environments, with an optional hollow wrist. Both Stericlean and Stericlean+ are available with a dedicated documentation package for full traceability and validation process support.
At MD&M West, Stäubli Robotics will demonstrate the robots’ capabilities in medical device manufacturing. A high-precision TX2-60 Stericlean robot paired with an Asyril flexible feeding platform will pick and place medical device components. The Asyril uses AI-based vision to locate acceptable parts and transmit their pick coordinates to the robot. Production is tracked by means of a monitor, allowing for traceability and process improvement.
Stäubli Robotics will also demonstrate how its technology enhances safety, accuracy, and efficiency in the operating room. A second demo will feature the TX2-60L, an ISO 14644-1 Class 2 cleanroom robot with hand-guided functionality for use in delicate procedures. Experts will present a use case for the anthropomorphic robotic arm in spinal surgery, showing how it uses a simulated navigation system to ensure the precise placement of surgical tools.