Marchesini Group introduces the BL A421 from the BL range of carton labeling and tracking machines. BL A421 offers new features to extend the range of sizes that can be processed without affecting the printing quality of 2D codes. Due to the unit’s versatility, customers can request a machine tailored specifically for their country’s product and regulation needs.
The BL A421, in line with the horizontal case packer and palletizer MC 820, is capable of labeling, tracing, and packaging 400 cartons/min. At the infeed, the cartons are gathered on an accumulation belt, after which a patented system indexes the cartons on a toothed conveyor belt. The system ensures positive motion and avoids cartons slipping by holding them still to guarantee correct label application.
The cartons are spaced apart by a patented mechanical system with just size changeover adjustments. The label dispensers are controlled by servomotors whose speed is automatically synchronized with that of the other motors in order to optimize the production process. Once the cartons are under the labeling heads, which have very fine vertical and horizontal adjustment mechanisms, they are labeled with a vignette on the upper panel and with two tamper-evident seals on the closing points.
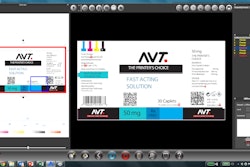
The 2D Data Matrix code is printed on the upper panel and on the side flaps to identify every single carton as well as the human-readable data. These codes are then verified by a camera. The machine is managed with Fail Safe logic so that any non-compliant products are rejected at the end of the process. All of the main tracking devices, such as ink-jet and laser printers plus relevant cameras, can be installed on the machine to print and verify (on the monitor) the serialized codes and related human-readable characters on the side flaps of the carton and on the upper panel.