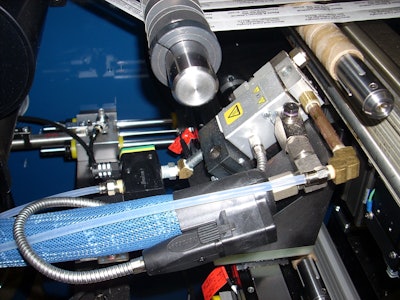
CTC International introduces a programmable spray core glue system for its turret rewinder that replaces the core glue pot with a traversing nozzle to apply glue to the cores on a rotating spindle. Glue application is via a precision spray nozzle. The stroke, beginning and end points of the glue nozzle traverse, are controlled through the PLC.
The programmable spray core glue system eliminates the need for multiple core glue wheels and the issues associated with them, including having to move them when the web stream width changes.
The system also provides longer turret runs before having to add more core glue, as it includes a large tank and pump supplying the glue to the applicator nozzle.
Another benefit is decreased job changeover times for turret rewinders as there are no glue wheels to move.
The spray glue system can be programmed to provide a gap in the spray pattern so that adjacent cores are not stuck together. It can also be programmed not to apply glue in locations where materials are narrower than the core width.
Like CTC’s Doctor Blade Glue Wheels, this spray system maintains the rewinders’ capability to deliver the last label cleanly off the core without glue residue.






















