This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
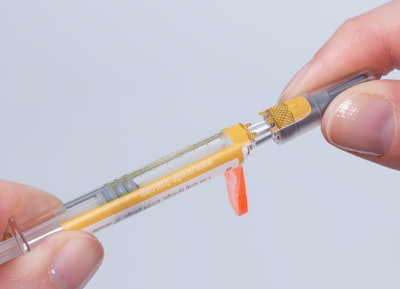
Schreiner MediPharm will introduce a new first-opening indication for its Needle-Trap solution at Pharmapack Paris, February 1-2. At Booth D46 in Hall 7.2, the company will debut a special security seal integrated into Needle-Trap that reliably indicates whether a prefilled syringe has been previously opened, helping protect the integrity of its contents until final use.
The Needle-Trap solution’s closure seal has a tab ending on the syringe cap. Before injection, the needle trap is folded sideways. While the cap is being pulled off, a perforation automatically activates the label-integrated seal, which irreversibly indicates the label’s initial opening. Special security cuts prevent undetected removal of the seal. For authenticity verification, overt authentication features, such as a guilloche pattern or covert security features, can be added.
Needle-Trap’s new feature provides pharmaceutical manufacturers with a multifunctional and cost-efficient solution, combining protection of healthcare staff against needlestick injuries with first-opening indication that ensures unit-level prefilled syringe integrity. Healthcare staff benefit from efficient and reliable needle protection, as well as convenient first-opening indication that is irreversible and detectable at first glance, enhancing both product and patient safety. In addition, the new Needle-Trap version can be easily integrated into existing pharmaceutical manufacturing processes.





















