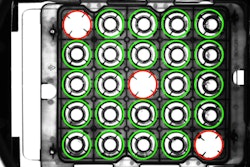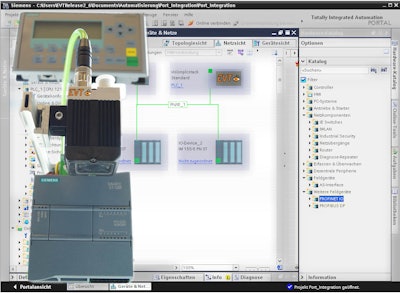
EVT Eye Vision Technology’s EyeVision 3.1.12 LTS offers revised color inspection commands and an extended Code Reader (DMC, barcode, QR, etc.). The complete EyeVision command set is available for the user, and therefore the system can be used for measurement technology, code reading, OCR/OCV, object detection, surface and color inspection, pattern matching, and 3D and Robot Vision. With the software’s graphical user interface (GUI) the inspection programs can be put together by selecting the graphical tools and commands, just like selecting apps on a smartphone.
Additionally, the new EyeVision 3.1.12 has more commands for 3D measurement and also more filter commands available. Users will find that the toolbox is better arranged as well as the new plug-in for creating their own commands. With the command plug-in, self-arranged commands can be integrated into the EyeVision GUI, which simplifies the process of putting together an inspection program.
The EyeVision software is available in English, French, Spanish, Chinese, Korean, Japanese, Russian, and Turkish.