This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
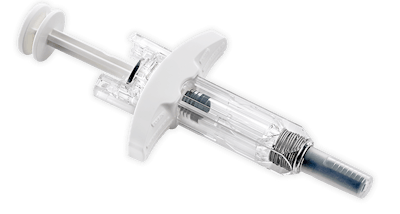
BD (Becton, Dickinson and Company), a global medical technology company, announced the launch of the BD UltraSafe Plus™ 2.25 mL Passive Needle Guard for use by pharmaceutical companies in drug-device combination products. When combined with a Glass Prefillable Syringe, the BD UltraSafe Plus™ 2.25 mL system enables the subcutaneous delivery of biologic solutions of different fill volumes up to 2 mL and viscosities up to 30 cP.
BD UltraSafe Plus™ 2.25 mL is the latest solution to be commercially released into BD’s portfolio of drug delivery systems for combination products and is designed to meet the needs of healthcare providers, patients, and caregivers in performing manual injections of biologic solutions. The design of BD UltraSafe Plus™ 2.25 mL complements bio-pharmaceutical companies’ combination product strategies – enabling patient-controlled injection for complex, high-viscosity drugs.
Biologic therapies are often self-administered by patients or caregivers and require delivery systems that provide ease of use and safety benefits for use in non-clinical settings. However, the viscosity and injection volume of biologics has shown a tendency to increase over the last years (>1 mL, >10 cP) that are beyond the capabilities of many commercialized injectable drug delivery systems. Biologic formulations with high volumes and viscosities often require stronger forces to inject, which can create challenges from a user’s perspective.
“With BD UltraSafe Plus™ 2.25 mL, we are innovating drug delivery systems with a goal to provide confidence and ease of use to patients and improve their self-injection experience, and to serve the expanding biologic drug delivery design space,” said Eric Borin, worldwide president, BD Medical – Pharmaceutical Systems. ”1
“We are excited to support bio-pharmaceutical drug launches with a new and robust platform technology that is compatible with BD Neopak™ 2.25 mL Glass Prefillable Syringe and supports our customers’ high-speed assembly by leveraging extensive experience with BD UltraSafe™ Passive Needle Guard in commercialized combination products.”
In developing a patient-centric solution, BD UltraSafe Plus™ 2.25 mL was evaluated in a human factors validation study in which usability was demonstrated and the majority of participants expressed confidence that the activated safety mechanism would protect them from needlestick injuries. BD UltraSafe Plus™ 2.25 mL Passive Needle Guard is now available for development by bio-pharmaceutical companies.