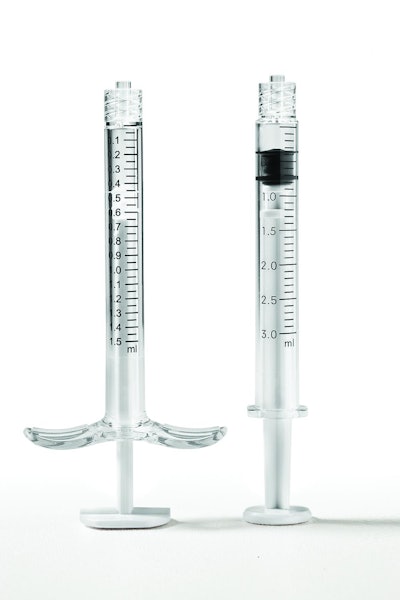
Plas-Tech Engineering Inc., a custom molder of medical devices and diagnostic components, formed Equinox Medical LLC, a stand-alone business entity that manufactures pre-filled syringes for pharmaceutical and biotechnology companies.
Plas-Tech Engineering formed the new company in response to customer demand from emerging biotech and drug companies for a low-volume syringe supplier that offers flexible and customizable solutions.
For those companies in early-stage product development and clinical trials, Equinox pre-filled syringes provide an option. For drug developers that require pre-filled syringes, the companies deliver fast speed-to-market in order to facilitate validation and commercial introduction.
Equinox offers pre-filled syringes ranging in size from 0.5-mL to 3-mL for drug delivery of insulin, diabetes drugs, allergy medications, and ophthalmic solutions. Pre-filled syringes made of cyclic olefin copolymer (COC) from TOPAS Advanced Polymers replace glass and enhance performance over other syringe materials.
These injection-moldedsyringes offer exceptional moisture barrier and chemical resistance, along with excellent clarity. The moisture barrier of COC syringes extends the shelf life of some pharmaceutical solutions over three years, said to represent an achievement that can’t be met by competing thermoplastics like polycarbonate or polypropylene which are used today in less demanding syringe applications, according to Equinox.The drug maintains its purity because COC is biologically inert with very low extractables. COC resin also offers excellent biocompatibility and meets the requirements of U.S. Pharmacopoeia Class VI and ISO 10993.
COC eliminates breakage problems during filling, in shipping, and at point of care. These syringes offer unique molded-in design features, thus providing greater part consolidation than more costly multi-piece glass assemblies. Molded COC syringes also give pharmaceutical companies shorter lead times than glass, allowing them greater flexibility in testing.
The push to eliminate silicone in the plunger tip and push rod has resulted in the development of Equinox’s silicone-free syringe option that has zero break force, low newton glide, and the highest chemical resistance for use with many new drug formulations. The Equinox syringe also comes with other options including private-label branding, a patented tamper-evident sharps container, and customized packaging to meet OEM needs.
Equinox has already sold pre-filled syringes to drug and biotech companies for aesthetics (i.e., botox and HA injections), ocular, orthopedics, and diabetes care applications. The company is currently developing pre-filled syringes in 5-ml and 10-ml sizes.