Editor’s note: Innovation is the lifeblood of package development. Interpack 2014 was awash in new and innovative packaging technologies. To cover the international trade show, Packaging World magazine unleashed a special team of editors and contributing editors to comb the aisles of the Düsseldorf (Germany) Exhibition Center and report back on the packaging technologies they found most intriguing. The team included Packaging World VP/Editor Pat Reynolds and Senior Editor Anne Marie Mohan, as well as five distinguished packaging professionals from Packaging Technology Integrated Solutions: Mike Richmond, Brian Wagner, Jill Ahern, Peter Menary, Phil McKiernan, and Jocelyne Ehret. What follows are individual developments related to pharmaceutical and medical device packaging. Read their complete interpack 2014 report on packaging developments across all industries. The next interpack event will be held May 4-10, 2017, also at the Düsseldorf Exhibition Center.
Active and intelligent packaging
A research center in Spain specializing in packaging, ITENE has more than 90 technical resources focused on packaging research topics. Their goal is to generate scientific and technical knowledge in packaging, and they have a particular focus in active and intelligent packaging. They have identified three specific areas of focus in this area, including bio-nano composites (nano clays) that disperse into the matrix of biopolymers (PLA). Results indicate barrier enhancement by 20% to 40%.—Mike Richmond
Cool new portion packs
Easysnap Group, Bologna, Italy, displayed their “One-Hand Opening Sachet,” which they view as the only significant step change in portion-pack containers over the last 20 years. The company also builds the machinery to produce the Easysnap sachet and can provide co-packing services for clients. The Easysnap single-dose pack is designed to replace conventional portion-pack tear-top flexible sachets, small bottles, monodose containers, and thermoformed cups.
It’s certified for use with food, medical device, industrial, and cosmetic industries applications. The key concept or convenience is the ability to open and dispense product using only one hand. The Easysnap sachet is held in one hand and then folded with two fingers. When the sachet is folded, a mechanically made center cut (using a patented process) breaks open progressively, allowing the product to flow out in a controlled manner.
These sachets are available in sizes from 1 to 30 mL (possibly larger depending on application). The device has received all major food safety, medical device, and cosmetics certifications. Some examples of food applications are ketchup, salad dressings, syrups, and energizing liquids. Medical and cosmetic applications include liquid medications, ointments, baby care products, hand sanitizer, sun screen, and moisturizer. The Easysnap technology also offers extended shelf life vs. traditional sachet packaging. —Peter Menary
Snapsil is back
Several years ago we reported on Snapsil®, a line of snap-to-open patented portion-control containers for a wide range of liquid or dry products that made it possible to open and dispense product with just one hand. Snapsil® seems to have new life, with a move from injection molding to thermoforming through partnership with Multivac, Bemis®, Klöckner Pentaplast, Visy, Sealed Air, THEM, and others.
As we have seen with the success of stick packs in the U.S., THEM has a unique ability to drive marketplace success through collaboration with material, machinery, and contract manufacturing partners. Monolayer PP as well as multilayer structures are available. According to Snapsil’s CEO Neil Cashman, developments with PET are underway. —Brian Wagner
Novel retort for pouches
Unlike retort systems for metal cans that are fundamentally continuous-motion in nature, most retort systems for flexible packages are batch systems. They require a lot of handling or “touches.” Moreover, there are limitations on the size of the trays that hold the pouches as they go through the retort, and the pouches typically need to be all of one size.
Steritech has developed SerialTower, a twin-tower retort system for flexible pouches that gets around many of these previously unavoidable limitations. It takes pouches vertically up through a heating tower and then down through an adjacent cooling tower. The pouches are automatically loaded into retort trays and unloaded out of these trays for handling by secondary packaging equipment. So it eliminates many of the loading, unloading, handling, and waiting steps that have traditionally kept the retorting of pouches from being an efficient operation.
With pouches automatically loaded from primary packaging lines, retorted, and returned to secondary packaging lines, all kinds of manufacturing efficiencies and product quality advantages are gained. Finally, by avoiding having to heat and cool everything with each batch, SerialTower reduces costs on energy and water consumption every year it’s in use. The SerialTower system can handle 4 tons per hour, and Steritech claims a 30% shorter cycle time than batch retorts.—Mike Richmond
Modular system for pharmaceuticals
Elsewhere, this time in the area of pharmaceuticals, Optima Packaging Group GmbH presented its INOVA SV modular system, which covers a wide spectrum of applications, from testing to small and medium-sized batches. Notes Optima, because biopharmaceutical products have special requirements, numerous product changes are typical. The INOVA SV can be equipped with upstream and downstream equipment and barrier functions for safe processing of various types and classes of pharmaceuticals. The system allows for the processing of nested ready-to-use syringes, nested vials, and ampules in one unit. A range of filling systems can be used, including rotary piston pumps, peristaltic pumps, mass flow metering, and time-pressure systems. Options also exist for the number of filling heads, the dosing range, buffer systems, upstream handling, closing systems, and barrier systems. —Anne Marie Mohan
Anti-counterfeiting label & cap combo
A new tool in the pharmaceutical manufacturer’s anti-counterfeit packaging toolbox, the Flexi-Cap label-and-cap combination from Schreiner MediPharm provides clear, irreversible tamper-evidence to prevent the illegal reuse of medicine containers under the guise of being unopened, original products.
According to Schreiner Marketing Communications Manager Hildegard Mock, the idea for Flexi-Cap was adapted from wine-bottle capsules and tailored to the specific requirements of the pharmaceutical industry. To create a package with the Flexi-Cap, a film cap, or “capsule,” is positioned over the closed container. Then, the label is applied without covering the peel-open tab of the opening strip on the film cap. Once the strip is opened, the bottom part of the cap, together with the label, remains attached to the container. Attempting to remove the rest of the cap destroys the label, eliminating the possibility of unnoticed illegal reuse.
As its name implies, Flexi-Cap is flexible, accommodating different container types, forms, and sizes. Because it does not require heat for application, it is a viable solution for temperature-sensitive medicines. Flexi-Cap can also be easily integrated into existing label dispensing processes. To further ensure brand security, users can combine Flexi-Cap with other brand protection features, such as holograms and color-shifting inks. The top of the film cap provides space for barcode printing or for NFC chip integration for electronic tracking. Flexi-Cap can also provide special functionality, such as extended-text labels. —Anne Marie Mohan
Induction integrity verification system
I2VS from DIR Technologies is the first commercialized thermal imaging inspection system for induction seals. The portable scanning equipment uses real-time quality inspection to facilitate process validation, control, and verification as outlined in 2001 FDA Stage 3 process validation guidance. At run speeds of 300 bottles/min with the cap on, I2VS will identify and remove bottles suffering from the most common induction-seal problems, including bent foil, missing foil, under heating (cold soldering), overheating, crooked cap, and laid-down or upside-down bottle.
This new inspection system enhances quality by inspecting each bottle/seal/cap produced, thus eliminating batch inspection systems using traditional water bath test equipment. Additionally, each bottle is imaged using sophisticated infrared detectors and thermal imaging. Data and reports generated are compliant with 21-CFR-11 requirements. Pricing was identified at approximately $200,000 U.S. —Phil McKiernan
Automatic EOAT changeover
An impressive four-robot wraparound cartoning system from Gerhard Schubert GmbH drew lots of attention at interpack. One robot was for erecting the carton, a second loaded bottles into the carton, a third closed the carton, and a fourth picked the finished carton and placed it on a discharge conveyor.
The most impressive aspect of this machine is that, should the end user need to go to a new bottle size, the end-of arm tooling can be changed in a matter of minutes with no more human intervention than the press of a button at the HMI screen. The key is a central transmodule device. When containers are being cartoned, this is the device that carries them through each step of cartoning. But when it’s time for a changeover, this device has automatically attached to it an adapter that becomes the carrier of the end-of-arm tooling. It positions itself in front of each robot so that the robots can rid themselves of the tooling that needs to be removed and stored. Each tool is then placed, again without human intervention, in a cabinet at one end of the long machine. Once all the tooling that needs to be replaced has been removed and stored, the transmodule device then picks up the new end-of-arm tools from another storage cabinet and methodically carries them to their respective robots so that robot and tooling can be joined. When all the new tools are in place, the system automatically removes the adapter from the transmodule and the transmodule resumes its task of transporting containers through the cartoning process. —Pat Reynolds
'Backing paper-free' labels
There’s lots of buzz of lately regarding linerless label concepts. Avery Dennison’s new LPA 81x print-and-apply labeler with LightSmart™ “backing paper-free” material. According to an Avery Dennison sales rep at the interpack booth, “The glue is the innovation.” Rather than using a sticky adhesive on the back of the label that can gum up the printer, LightSmart material is coated on the reverse side with a non-tack adhesive. Using the LPA 81x print-and-apply unit, data is printed on the material, and labels are cut to the desired length. The adhesive backing is then activated through infrared heat. The LightSmart label becomes sticky through heat activation after the print-and-cut state—a key differentiator compared to existing backing paper-free print-and-apply solutions, says the company. The sticky label is then applied to a carton, tray, or pallet.
As Avery Dennison explained at interpack, LightSmart material allows for 60% more labels per roll versus conventional pressure-sensitive labels with backing paper, resulting in reduced inventory space, reduced shipping costs, and fewer roll changes during operation. The lack of backing paper also eliminates waste as well as the time and cost associated with waste handling. Waste is also eliminated due to the printer’s ability to change label lengths on-the-fly. Among the environmental advantages cited by Avery Dennison of LightSmart compared with conventional p-s labels are the following: a 47% reduction in water usage; a 25% reduction in energy; a 2% reduction in the carbon footprint; and a 65% reduction in the solid waste footprint. LightSmart technology was a joint development with the Fasson division of Avery Dennison. —Anne Marie Mohan
Thermoformable paper barrier tray
PaperLite® sustainable packaging solution produced by Flextrus, a member of the AR Packaging Group, brings the benefits of paper-based packaging to the deli category. PaperLite is an FSC-certified paper-based, thermoformable base web that allows for both a reduction of plastic and a unique paper look and feel for consumers. According to Flextrus, the secret to PaperLite is that it’s a special grade of thermoformable paper that has barrier properties added to it in the form of a coextrusion coating of PE/tie/EVOH/tie/PE. The material is also suitable for heat sealing lidstock to it. Current commercialized applications include sliced cheeses and meats, but PaperLite is suitable for many other shallow tray applications, as well.
Options to customize the technology include easy-peel lidding and both medium- and high-barrier protection grades. Business Development Manager Ronny Gimbe shared the technology benefits, which include a strong sustainability story from reduced packaging weight compared to standard polymer-based materials, environmental benefits since the source of PaperLite is FSC-certified sustainably managed forests, and recyclability under EU regulations since the barrier represents just 14 to 15% of package weight. In addition to sustainability benefits, PaperLite meets the growing consumer demand for premium product packaging, including high-quality flexo printing, advanced graphic reproductions, and a unique paper tactile quality. PaperLite provides an opportunity to differentiate products on-shelf and appeal to consumers who prefer fiber-based packaging and a premium or artisan brand “halo.” Expect to see continuous evolution of examples like this where packaging technologies balance consumer demand and high performance. —Jill Ahern
High-heat-application bio resin
Newly introduced by Biotec at interpack, BIOPLAST 900 is a plasticizer-free thermoplastic material that contains 69% biologically sourced polymers such as PLA and potato starch. The material is suitable for high-heat and short-cycle injection molding processing as well as for conversion by sheet and blown film extrusion. The material is mainly designed for packaging dry and/or fatty foods; application for hot-filling beverages is another option. At interpack, a k-cup for coffee was shown with claims of complete biodegradability according to EN 13432; compostability (depending on thickness) and allowance for incineration were also claimed.
Additional general applications include injection-molded articles (cutlery, medical devices, clips, cups for hot and cold drinks), thermoformed products such as food trays, and blending with other resins. Formed products can be printed by flexographic and offset printing without pre-treatment. —Phil McKiernan
Progress in renewable resources
While development of packaging materials made from renewable resources has been in the works for several years, at interpack there were new examples of potential breakthroughs for packaging applications with sustainable sourcing being taken to new levels. Whole new sources are emerging. Notable are the ones that don’t compete for feedstock, have a compelling performance advantage, or are otherwise advantageous over current technologies. Two intriguing examples of such research are currently being developed by collaborations funded under the European Commission’s Seventh Framework Programme. The first, the OliPHA, is a three-year consortium-funded R&D study currently evaluating the development of a method to produce polyhydroxyalkanoate polymer-based packaging from widely available olive-oil production waste water. The research team believes that PHAs have great potential to replace many plastics used in packaging today, and they are seeking more cost effective methods of production and sustainable PHAs.
The second is WheyLayer™, a three-year R&D project that began in 2008 and now is entering the scale-up and patent review phase. It aims to replace currently used synthetic oxygen-barrier layers with whey protein-coated layers readily available as a by-product of food processing; these materials are often discarded in the EU. At present, pilot production is underway for testing various materials and market applications, including use in food, pharmaceutical, and personal care. —Jill Ahern
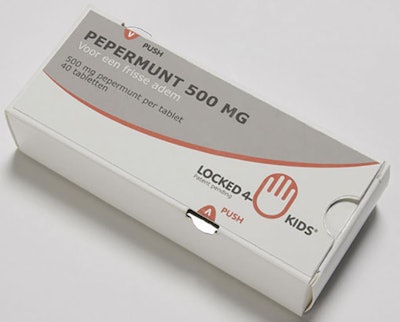
Child-resistant pharmaceutical carton
Packaging that brings benefits on several fronts is always a winner, especially when the benefits include increased sustainability and lower cost. Locked4Kids is that kind of packaging.
Ron Linssen, Managing Director of Netherlands-headquartered EcoBliss was on hand at interpack for the press event announcing Locked4Kids. Linssen explained that as a manufacturer of blister packaging for pharmaceuticals, EcoBliss was keenly aware of the continued threat of children being poisoned by household materials. That’s what led to the creation of the patented Locked4Kids recloseable carton, which meets both the European and US standards for child-resistant pharmaceutical packaging. The package design consists of a tear-resistant outer carton with a locking blister tray inside. Slots on the edges of the carton align with hooks on the blister tray. In order to remove the tray, hooks near opposite corners of the package must be simultaneously pressed, a task nearly impossible for a child’s hand to execute. Adults, however, are able to press and slide the tray quite easily. Locked4Kids reduces the high cost and additional material required for many other child-resistant packages, while still maintaining the sizeable face panel and stackability of a carton. Locked4Kids was nominated as a Top 10 finalist in the De Gouden Noot 2014 Packaging Innovation Awards. Watch for commercial applications of this innovation to be introduced in the coming year. —Jill Ahern
Novel dosing system
Dispensing closures for liquid and dry products can be messy. Overdosing is reported to happen more than a third of the time. Menshen has developed a new two-part system with a flip-top cap solution to get rid of the mess and add convenience for consumer use. The idea is simple with the dosing or charging put into the bottle neck area. The package is turned upside down to charge the one2dose® system for the first time. When turned upside down (after the first time) the bottle discharges the appropriate dose. The accuracy is reported to be plus/minus 10%, which means significant product is saved over time. Menshen notes that this system will be introduced in North America in 2014 in the laundry sector with a 20-mL dosing charge. —Mike Richmond