This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
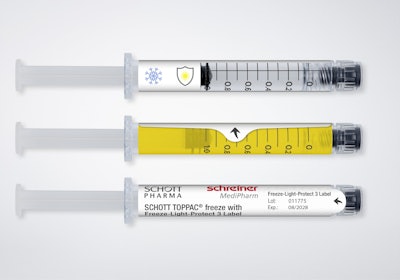
The Schreiner MediPharm Freeze-Light-Protect syringe label combines light protection with viability at temperatures well below zero. The new solution is particularly relevant for sensitive mRNA-based substances, as well as cell and gene therapies.
The Freeze-Light-Protect label securely adheres to the syringe, enabling reliable marking, despite extremely low temperatures. The specialty label also can be enhanced with a variety of UV and light protection levels. The first level provides UV protection exclusively, while the second protects against UV rays and blue light. A third level delivers comprehensive light protection. Light protection levels can be customized to suit the specific requirements of the active ingredient.
Additional integrated functionalities are available, including detachable documentation labels for vaccination cards or patients’ medical records; inspection windows for checking syringe contents in unadulterated color; and graduations for correct dosing of injections.
Schreiner MediPharm will showcase its Freeze-Light-Protect syringe label at PDA Universe of Pre-Filled Syringes & Injection Devices, Booth #135, October 17-18 in Gothenburg, Sweden.






















