This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
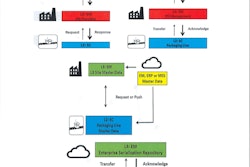
The Healthcare Distribution Alliance (HDA), in collaboration with ValueCentric, LLC, today announced the official launch of Origin: HDA’s Product Data Source. Origin is a high-powered, central data repository hosting verified Global Trade Item Number (GTIN) information. The service enables the secure, accurate and efficient exchange of pharmaceutical product master data between manufacturers and their supply chain partners to help comply with the Drug Supply Chain Security Act (DSCSA).
“From inception to the launch today, Origin is the result of hard and important work by the industry, for the industry,” said Perry Fri, Executive Vice President, Industry Relations, Membership and Education, HDA. “The product effectively eliminates the need for repetitive communication and allows data sharing through an easily accessible, cloud-based portal. It also sets the pace for good data management practices that will serve the industry well as it implements a long-term traceability plan.”
Origin’s launch follows a successful evaluation period, which demonstrated the system’s ease of use and value. Product testing was completed by organizations representing stakeholders from across the industry including five manufacturers, three distributors and one dispenser. More than 550 active GTINs are active in the system. To date, all participants readily have seen the value of the central repository to help limit multiple one-to-one communications about individual product master data.
Companies interested in subscribing to this service may visit the product page, www.hdaorigin.com, to enter their information and receive a custom quote. The webpage includes registration information, and such resources as a product fact sheet and a checklist to prepare for Origin participation.