This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
CEIA Launches Advanced THS/PH210® Metal Detector Series with Full FDA Compliance
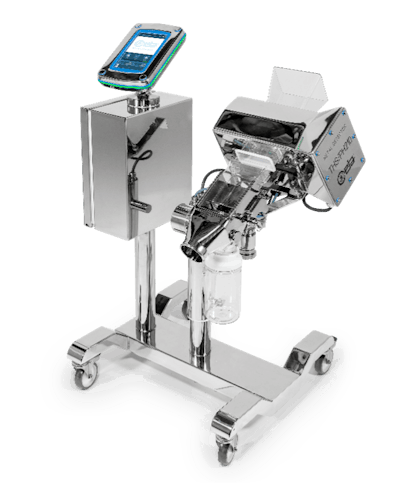
CEIA, a global expert in industrial and in pharmaceutical inspection technologies, is pleased to introduce its latest advancements in metal detection: the THS/PH210 Series metal detectors. These cutting-edge systems are fully compliant with FDA 21 CFR Part 11 and FDA 21 CFR 210, delivering unparalleled performance, security, and traceability in pharmaceutical applications.
FDA 21 CFR Part 11 Compliance for Data Integrity and Traceability
The THS/PH210 Series metal detectors meet the stringent criteria outlined in FDA 21 CFR Part 11, ensuring that user access, data protection, and traceability are maintained at the highest standard. User access is secured through a User Name and Password login system, managed by a System Administrator who controls individual user profiles. This ensures only authorized personnel can access the system, safeguarding the integrity and the security of operations.
Additionally, all events, including metal detections, ejections, program operations, and calibration tests, are logged to assist with compliance and root cause analysis. This comprehensive logging mechanism is protected by a proprietary checksum algorithm to guarantee data integrity and prevent tampering, ensuring a robust solution for regulated environments.
FDA 21 CFR 210 Compliance for Good Manufacturing Practices (GMP)
The THS/PH210 Series are also fully compliant with FDA 21 CFR 210, adhering to stringent good manufacturing practices (GMP). These systems are designed to detect and to eliminate metal contaminants in pharmaceutical products, ensuring that drugs meet the highest quality and purity standards. These systems utilize advanced sensors to verify the successful ejection of contaminated products, offering a reliable and traceable solution for pharmaceutical manufacturing.
State-of-the-Art Features for Maximum Performance
CEIA’s THS/PH210 metal detectors provide an array of innovative features, enabling pharmaceutical manufacturers to achieve the highest levels of performance and compliance:
- Fully Digital Structure: The systems employ a fully digital internal structure, replacing analog processing with numerical digital processing, eliminating the need for manual trimmer calibrations.
- Automatic Antenna Balance: A dynamic balance tracking system ensures optimal detection performance, regardless of environmental changes.
- Continuous AUTOTEST Function: Embedded in the system is a continuous AUTOTEST function, which verifies performance stability and consistency by comparing real-time data to reference values stored during the factory acceptance test (FAT).
- AUTO-QC Function: The AUTO-QC function automates quality control processes, verifying the activation of alarms and of ejectors through automatic and manual test stimuli, customizable according to user quality procedures.
- Multi-Point Auto-Learn System: This proprietary feature optimizes detection sensitivity to all metals, significantly reducing the time required for learning transits while improving precision.
Ensuring Compliance and Flexibility in a Changing Environment
CEIA’s THS/PH210 metal detectors are designed to maintain optimal performance across varying environmental conditions, thanks to their continuous auto-diagnostic capabilities. These systems can signal detected faults within fractions of a second, reducing downtime and improving operational efficiency.
Moreover, the systems support a wide range of communication protocols, including EtherNetIP, Profinet, Profibus, Ethercat, ModBus/TCP, and Profinet-OPC-UA, allowing seamless integration into existing pharmaceutical production lines. An optional embedded field-bus controller enables OEM system integration, maximizing configurability and ensuring the metal detectors meet the specific needs of each user.
ISO 9001 Certified Quality
Manufactured under CEIA’s ISO 9001 Certified Quality Management System, the THS/PH210 Metal Detection Systems consistently deliver products and services that meet or exceed customer and regulatory requirements. This certification further underscores CEIA’s commitment to delivering high-quality, reliable, and compliant metal detection solutions.
Key Benefits and Features
- More than 50% sensitivity improvement in detectable metal volume
- Easy, flexible programming for 500 product profiles
- Full AISI 316L stainless steel construction for maximum durability and sanitation
- Data integrity verification for 1,000,000 storable events
- Optional Level Sensor on Reject Bin, with alerts for full and absent bins
Revolutionizing Pharmaceutical Inspection
With the launch of the THS/PH210 Series, CEIA continues to set the standard for pharmaceutical metal detection technology. These systems offer maximum sensitivity, ease of use, and full regulatory compliance, empowering manufacturers to safeguard their products and maintain the highest quality standards.
Innovative Valve System for Pharmaceutical Inspection Applications: CEIA THS/PH210®-FFV
 THS/PH210-FFV Valve System
THS/PH210-FFV Valve System
Meeting the Unique Challenges of the Pharmaceutical Industry
Pharmaceutical production frequently involves small batches and multiple raw materials, necessitating regular and thorough equipment cleaning. The THS/PH210-FFV metal detector with valve system has been designed to optimize these processes by offering superior inspection and sanitation capabilities, ensuring compliance with hygiene standards, and enhancing production efficiency.
Key Features of the THS/PH210-FFV Valve System:
- Separate Flow Paths for Good and Contaminated Product The valve system features dual surfaces for product flow, with distinct paths for good and contaminated product, ensuring there is no cross-contamination during the switching process. This design innovation addresses a critical gap in existing market offerings.
- Monolithic Rotor Design Made without welds or joints, the rotor provides unmatched resistance and durability. This design also improves precision and facilitates better sanitation, ensuring high operational reliability.
- Open Semicircular Rotor Chambers The rotor’s unique design includes open, semicircular sections that simplify cleaning and inspection, enhancing the overall hygiene of the system.
- AISI 316L Stainless Steel Construction Both the rotor and casing are constructed from high-quality AISI 316L stainless steel, featuring a mirror-polished finish. This material choice ensures durability, resistance to corrosion, and optimal sanitation for pharmaceutical applications.
- Streamlined, Compact Design The valve’s compact structure eliminates changes in section between the product inlet and the product outlet, promoting smooth material flow and reducing potential product accumulation points.
- Tool-Free Disassembly With tri-clamp connections, the THS/PH210-FFV offers easy, tool-free disassembly and reassembly, allowing quick sanitization between production batches, which is essential for processes involving frequent raw material changes.
- Contamination Prevention By eliminating cavities, holes, and threads in product-passage areas and avoiding bolts in critical regions, the system prevents accumulation of processed material and reduces the risk of cross-contamination.
- No Sealing Gaskets in Product Path The absence of sealing gaskets between the rotor and the nozzles ensures no product waste accumulates, reducing the risk of wear and contamination while extending the system’s lifespan.
- Quick Product Expulsion With a rotor rotation angle of just 55°, the system ensures rapid and efficient expulsion of contaminated product, speeding up production cycles without compromising accuracy or safety.
- Minimal Surface Contact The design minimizes the surface area between static and moving parts, reducing the risk of product buildup and ensuring smooth, continuous operation.
Revolutionizing Pharmaceutical Production
The THS/PH210-FFV valve system sets a new standard for pharmaceutical inspection by addressing key pain points in the industry, such as cross-contamination, sanitation, and equipment efficiency. Designed to meet the most rigorous standards, it offers pharmaceutical manufacturers a reliable, easy-to-clean, and efficient solution that enhances overall production quality.
CEIA and Heat and Control can be found at PACK EXPO International 2024 in Booths N-6104 and N-6106.