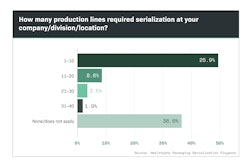
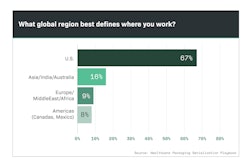
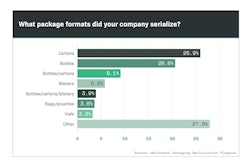
Although consultants, software providers and integrators were involved in serialization projects, the vast majority of manufacturers retained buying authority for line upgrades (Chart 6.1).
Although consultants, software providers and integrators were involved in serialization projects, the vast majority of manufacturers retained buying authority for line upgrades (Chart 6.1).
That may not be surprising, but it is noteworthy that 18.4% of respondents noted “other third party” to the question. To that, Dirk Rodgers, Founder of RxTrace.com, commented, “[These] could be readers who are virtual manufacturers and are dependent on their CMO/CPO to upgrade the packaging lines used for their product(s).”