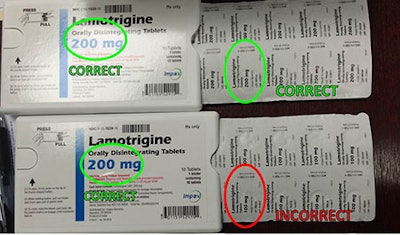
According to a recent FDA press release, Impax Laboratories, Inc. issued a voluntary recall of their Lamotrigine Orally Disintegrating Tablet (ODT). The August 19th recall involved incorrect labeling of their 10-count 200 mg blister packs. The blister cards within the unit-of-use blister packs are properly labeled as 100mg, but the plastic shell packs are labeled as 200 mg ODT.
The affected lot may contain 100 mg rather than 200 mg. This could result in consumers taking less than their intended lamotrigine dose, leading to “reduced therapeutic effects of lamotrigine and reemergence of epilepsy or bipolar disorder symptoms.” The notice asks consumers to carefully inspect their medication and report to affected doses.