This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
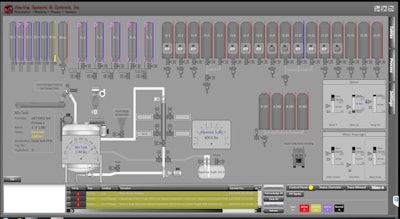
The Sterling Systems batching module is intended for the batching of solids and liquids. Capable of providing ingredient material traceability and batch validation, the BatchPro-SA batching automation and control application module can be incorporated into semi-automatic or automatic systems. Remote supervisory control using the Sterling Systems software app allows browser-based operation and report generation; exporting of data to integrate with corporate ERP systems is available. Each batching automation application is custom engineered.
Companies in this product