This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
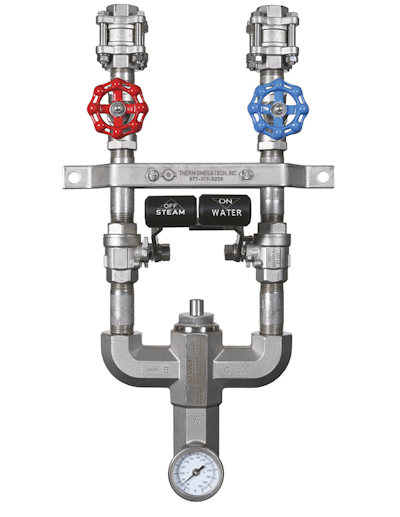
The ThermOmegaTech washdown station utilizes a silent-type Venturi mixing valve to mix steam and cold water inlet flow to produce hot water washdowns. If the output temperature exceeds the factory setpoint, the actuator will begin to reduce flow and completely shut down 15°F above it. Flow resumes once the output temperature falls below the shutoff temperature. If the cold water supply is interrupted, the STVM washdown station will automatically shut off and stop flow to prevent steam-only operation and resume flow once cold water has been restored.
In a time of considerable uncertainty, a constant you can count on is PACK EXPO International and Healthcare Packaging EXPO. PACK EXPO this November is taking place, either as a live and virtual event combination, or an all-virtual event. Learn more at https://www.packexpointernational.com.





















