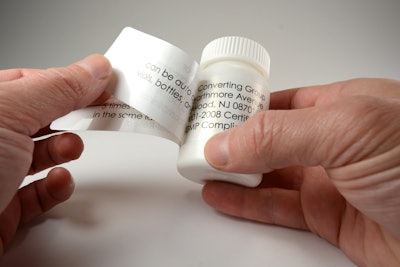
Luminer, a labeling systems provider specializing in extended content labels (ECLs) for pharmaceutical ethical and OTC packaging, clinical trials, and other narrow web applications for the cosmetics, F&B, and chemicals sectors, reports seeing a significant rise in demand for extended content labels for clinical trials—several of which deal with ongoing development for drugs related to the coronavirus pandemic. Another pharma niche seeing a spike in orders are labels for over-the-counter medications that mitigate flu-like symptoms. A number of these products, especially those in small packaging, require multi-page labels to meet regulatory requirements.
Extended content labels, or ECLs, are in especially high demand, as they are necessary for the sort of detailed multi-language patient instructions, strict regimen adherence, and tight oversight necessary for well-executed clinical trials. Luminer says it is well-positioned for this heightened emphasis: the company recently incorporated an additional ECL production line at its primary manufacturing facility in Lakewood, NJ. Highlighted by a custom engineered modular printing, converting, onserting servo platform, this infrastructure increased Luminer’s capacity, and yields additional benefits like faster throughput speeds and heightened booklet placement precision, says the company.
The equipment also helped Luminer expand its overall ECL capabilities portfolio. For example, the equipment’s modernized onserting and movable print and die stations are meant to help broaden production flexibility—a factor in executing the complicated and/or customized arrangements often utilized in clinical trials.
The other area seeing increased demand for pharma labels—medications that suppress flu-like symptoms—is an indicator of the sheer breadth of the COVID-19 pandemic. With over a million reported cases in America alone, the emphasis of such labels is tied to people showing coronavirus symptoms—most of which, fortunately, do not become ill enough to require hospitalization.
“It is undeniable that the coronavirus pandemic is playing a significant factor in determining which labels are in highest demand in the current pharma landscape,” says Tom Spina, president & CEO of Luminer. “The increased need for ECLs and labels for OTC flu-symptom suppressants has been noticeable for several weeks, and we expect it to continue into the summer.”