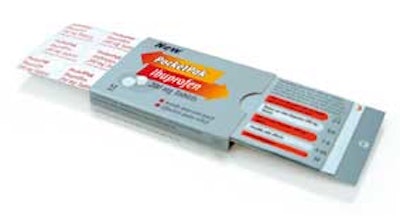
The PocketPak slides open by pulling on the notched area exposing the blister card on one side and a leaflet that provides consumer information and instructions on the other. It features space for usage, dosage, and labeling information. The leaflet is affixed to the paperboard, remaining with the pack while it's used, thus serving as a reference material. "The PocketPak was a stand-out entry that received ratings well above the other contenders," says Peter Mayberry, HCPC's executive director. "The judges were truly impressed with this package."
Judges at Interphex rated packages based on distinguishing qualities packs provide in terms of user-friendliness, package design innovation, unique features, and the ability to increase compliance.
The first runner-up in the trade package category was Amgen's Aranesp prefilled SureClick autoinjector. The combination device and drug is one unit, consisting of a spring-base, single-use and disposable product using a 1-mL prefilled syringe. It's designed to make drug delivery secure and simple for physicians and nurses. HCPC says it reduces medication errors because fewer preparation stages are required compared to vial and exposed needle manipulation. It is also said to protect against unintentional needle sticks. A DVD and pamphlet provide education and help drive drug compliance.
Earning second runner-up in the trade package category was Mylan Pharmaceuticals/UDL Laboratories' Clonidine Shellpack. A MeadWestvaco Healthcare Packaging compliance package, Shellpak provides child-resistance and a protective environment. It is a 30-count unit-dose pack with tablets arranged in four rows. "It's hard for kids to get to the blister [component of the package], but easy for adults to get the pills out" of the push-through backings, noted one judge.
In the category of innovative design for a pack not yet used commercially, judges awarded Cardinal Health's RxBarrier Plus. The F=1-rated child-resistant, senior friendly package incorporates a one-step opening process, is said to be cost-effective to manufacture, and its design promotes patient compliance. Developed by Cardinal Health's Packaging Services division, RxBarrier Plus is available for licensing.
HCPC is a not-for-profit trade association that promotes greater use of unit-dose blister and strip packaging. Complete competition guidelines are available at www.unitdose.org, or by calling HCPC at 703/538-4030.



















