Food and beverage manufacturers are constantly under pressure to improve productivity. So, what else is new? But many of these manufacturers admit they have never seen anything like the double whammy of SKU proliferation during pre-pandemic days and the supply chain disruptions over the past 18 months.
Prior to the pandemic, SKU proliferation brought on by ever-increasing consumer demand collided with the “thinning” of manufacturing operations, resulting in food manufacturers having to do more with less, putting unprecedented stress on their manufacturing operations. During the pandemic, failure to meet consumer demand has been exacerbated by worker shortages due to safety concerns, supply chain disruptions, materials and ingredients shortages, and a host of other issues, all contributing to unparalleled pressure on productivity.
To address these challenges and uncover ways that consumer packaged goods (CPG) companies can solve them, FSO Institute and its transformational partner GE Digital conducted two roundtables earlier this year with European and American processors, including more than 30 CPG manufacturers sharing their ideas. The European roundtable—led by panelists John Giles, Amway; Joe Zembas, The J.M. Smucker Company; and Bernard Cubizolles, GE Digital—focused on multifunctional equipment and changeovers. The Americas roundtable—led by panelists Jim Prunesti, Conagra Brands; James Couch, Smithfield Foods; and Cobus Van Heerden, GE Digital—focused on predictive process analytics.1
Following are some of the key takeaways from those roundtables.
Multifunctional equipment and changeovers
- Build a business case if you haven’t already. How a long run vs. shorter run strategy is justified isn’t always clear, so it’s critical to determine how to “cost” things, including what’s in the calculation and what’s not, and to get agreement on it. To do this, use cross-functional teams to build the business case to get broader input, better direction, and role clarity, as well as greater alignment by all. There should be a clear answer to the question as to how and why certain changeovers are better. Avoid too much complexity in explaining the business case—it should be readable, understandable, and manageable for all.
- Put a premium on agility. The ability to move quickly and easily—agility—is a common challenge in the manufacturing community. E-commerce has driven much of the need for agility in two key areas: operational improvement and flexible equipment. A good example of each is single-minute exchange of dies (SMED)—an operational tool that can substantially reduce changeover times and improve operational efficiency. Additionally, many CPGs are investing in end-of-line flexibility, where much of the late-stage differentiation in production occurs, especially in the e-commerce space.
- Automate, but steadily. An all-too-obvious solution to the tight labor market is automation, but deciding what to automate requires contemplation. Is it end-of-line, materials handling, receiving, or any of the other myriad areas that could be automated? Also, it is important to think about the skill sets required for newly automated areas, as they are often higher level than before and perhaps more difficult to find in the workforce. For many manufacturers, learning and adapting at a slower pace are part of the natural evolutionary process in adapting to change.
- Reduce downtime, speed up, and maintain quality. To reduce downtime, many CPGs are more consciously focusing on asset reliability to gain a better understanding of downtime causes and solutions while building the business case for its implementation. Quality focus is driven by continuing consumer demand, especially for awareness of product origin and a packaging format that is responsive to a sustainable, circular economy environment. To speed up, focus on process innovation and smaller operator-driven improvements by engaging the workforce—your internal experts—like never before.
- Rethink yield loss and what it means. Getting the most out of assets is a common aspiration for all manufacturers, and it’s generally accepted that the proliferation of SKUs will inevitably lead to yield loss. But a critical question to ask about changeovers in today’s environment is: Do you really need the extra capacity that the changeovers bring? Let your business case and its prescriptions guide you in answering this question, and don’t do it if you don’t have to or if your business plan isn’t met as a result of it. Why invest in a production line that is only marginally utilized? Consult your business case for guidance on this as well.
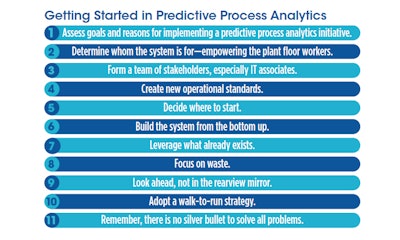
Predictive process analytics
- Build a compelling business case. Building a comprehensive, compelling business case is essential to beginning the journey to predictive process analytics.
- Why predictive process analytics matter. Getting better visibility into production operations, measuring losses, and realizing the true performance of assets (actual and possible) are primary targets for utilizing predictive process analytics.
- Tools are already available. The newest technologies (artificial intelligence, machine learning, edge devices, etc.) are available for implementation to help accelerate the predictive process analytics journey.
- Clarify goals and ask the right questions. Determining the goals of a predictive process analytics initiative helps in the formulation of the critical questions to ask.
- Start at the plant floor. Start with the plant floor operators, and build a team around them, then add the IT department and other critical stakeholders, to ensure the initiative’s success.
- Start with what you already have. Adopt a walk-to-run strategy by utilizing resources and systems already in place. Remember that the process is a journey that takes time—there are no silver bullets.
- Buy-in or bust. A predictive process analytics initiative that doesn’t resonate with plant-level associates is doomed to failure, as it is these associates who must buy in, own, and advocate for the system.
- Ease into it. Don’t move too fast or expect immediate results. Instead, look for incremental improvements, the results of which may fund future projects.
The most significant reasons for adopting predictive process analytics are:
- Using a visionary system will capture trends and performance and present information to allow for adjustments before an issue arises.
- Providing an opportunity to get better visibility on losses—small losses cumulate to much larger ones as greater visibility into them becomes available.
- Determining the true performance of assets measures the real cost of downtime (asset reliability) and performance improvement or overall equipment effectiveness (OEE).
- Interpreting the complexity and variability in production operations.
- Taking advantage of the newest technologies and the next evolution of equipment intelligence.
- Informing the timeline for improving production operations.
To download the full FSO Institute/GE Digital European and Americas Roundtable reports, go to www.fsoinstitute.com/tools.
1The participation of the industry panelists in these roundtables is for educational purposes only and does not represent an endorsement of GE Digital, its products, or any other platforms and resources used in their digital journey.