Simulation and virtual prototyping is becoming an important and indispensable stage in the development of robust, cost-effective temperature-controlled packaging, especially when project timeline pressures are a major factor. The packaging industry is now able to consider the use of the previously cost-prohibitive Multi-Physics (MP) technology used in the development of jet engines and Formula One cars for developing complex temperature-controlled solutions for its pharmaceutical clients.
The simulation capabilities of MP enable packaging suppliers to significantly increase the efficiency of a design process and allow for a more expansive and innovative exploration of solutions to meet customer requirements. In fact, a test using MP will take approximately one tenth of the time it would take to run a physical test in a laboratory.
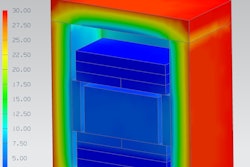
MP works by creating a 3D CAD model of the temperature-controlled packaging that is to be tested, which includes the various insulating materials, the temperature stabilizing components, and the product in the shipper, with temperature variables being associated with each part.
Traditionally, when developing and testing a new temperature-controlled packaging system for a client, packaging suppliers would undertake a number of iterative Design Qualification Tests, taking into account the transit duration time and variable temperatures it would expect to see during transport, in order to configure a shipping solution in the laboratory: a process, which undertaken in real time, could take months.
For example, in real time, if a laboratory test takes five days to perform, packaging suppliers will initially have to wait five days before they know whether or not their first configuration of a system will work. By using MP, a virtual Design Qualification Test is performed. Although the results are not relied on to be 100% accurate, they give a strong indication as to whether a packaging configuration will be good enough to move it to the laboratory for the physical Design Qualification Test. So, in essence, rather than undertaking a three-month development phase in the laboratory, three weeks can be taken. That condenses the development period, which enables suppliers to fulfil a customer’s requirements sooner.
MP enables temperature-controlled packaging suppliers to see how energy (heat) is transferring through a shipping system and the whole shipping system can be analyzed, including the insulation, coolant components, and product load area. Hot and cold spots can be identified and the flow of energy (heat) in and out of the shipper from external surfaces can be seen, identifying exactly how the external ambient environment is affecting it.
Furthermore, it can also give a complete picture of the solid/liquid phase of the coolant components in the shipping system, rather than (in a physical test) just seeing that the components have melted when a box has been opened after the test is finished. The software also simulates the phase change of the coolant components, showing the detail of a frozen (solid) component melting during the simulation into its liquid phase. This makes it possible to view the transition accurately to pinpoint the end of the phase-change period, which is critical when designing cost-effective shipping systems.
MP also provides an understanding of how to design the shipping system with exactly the right amount of energy-absorbing phase-change material to ensure internal payload temperatures are maintained within the correct range for the desired transit duration. This means that the old design method “safety buffer” can be removed, while still being confident that the shipping system is fit for purpose.
In fact, the more complex the shipping system, the more efficiency can be gained using MP: information can be obtained more quickly, development time shortened, and the products can be launched to market in a shorter timeframe.
As well as shortening the development time, the software can also simulate different styles of product that a customer might be shipping inside one temperature-controlled packaging system. For example, a specific temperature-controlled solution has been developed in the laboratory and signed off with one product type, such as freeze-dried powder. But how will the same shipper handle the transportation of another product, such as a liquid product inside a vial? How would changing the product affect the performance of the shipper, and how well would that configuration protect different styles of product being transported? The answers to these questions can be simulated by using the characteristics of each product type, tested against the previously qualified solution in the laboratory, thereby demonstrating a comparability of result without the need to re-run those real-time laboratory tests.
Ultimately, the availability of MP to this sector enables temperature-controlled packaging suppliers to provide their data quicker, more accurately, and with additional insights.
—Article provided by Richard Wood ([email protected]). Wood has worked in various design and manufacturing engineering functions during his career. He currently holds the position of Design Manager at DS Smith Plastics Cool Logistics, where he has worked since 2005. Wood has also helped to ensure that manufacturing practices are standardized across the Cool Logistics partners network. Richard’s current role within DS Smith Plastics Cool Logistics is focused on standardizing development, qualification, and manufacturing practices to help support the company’s global customer base.