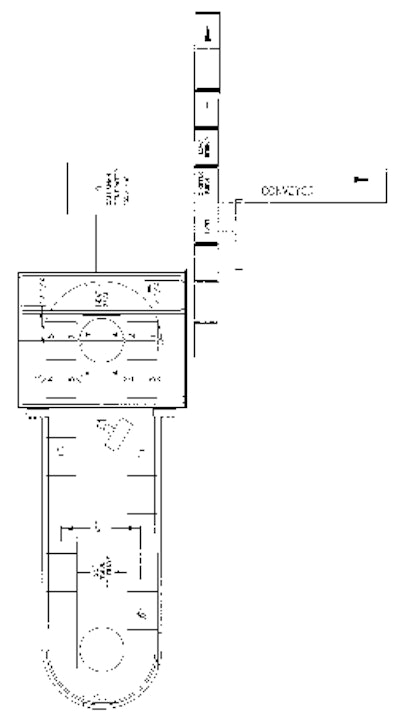
The new packaging lines at BIC’s plant in Greece are a bit of a hybrid for Alloyd. In essence, each line combines Alloyd’s 20-station blister packaging machine with a 12 ½-kW radio-frequency (RF) sealing station from Alloyd subsidiary, Callanan that replaces the heat-seal station. The blister machine itself provides for automatic feeding of both blisters and insert cards, while the product loading is done by hand.
The machine’s operation is described by Mike Osber, a BIC shaver packaging engineer: “We’re basically using just four of the automatic stations on the machine. At station one, the machine inserts the back blister into the nesting tray,” he says. After another station loads what BIC calls the body card, the machine cycles through the manual loading stations where the shavers themselves are inserted.
“At what we call station three, the header card is put in position, followed by the front blister at station four. At the next station, the packs move into the RF sealing station,” Osber says. When asked about how the finished packages are discharged from the machine, he points out that actually two operations are performed at the first station. Before the fresh blister is inserted into the nesting tray, another set of arms removes the freshly sealed packs and transfers them into pockets of what BIC calls the stripper conveyor. Also built and supplied by Alloyd, this conveyor carries the packages past a Label-Aire Model 2111CE blow-on applicator and to the stripper station that punches the trimmed package out of thePVC sheet.
“At the in-line trimming station, the punch or cutting head cuts the package away from its flange and the package drops onto an exit conveyor,” Osber says. The punch station is the device that allows the BIC pack to meet the dimensional needs of the displays in the retail stores. The exit conveyor takes the trimmed packs to a pack-off area where workers place individual packs into chipboard cartons that are then loaded into shipping cases.
Labeler requirements
While it’s important to point out that the automated lines require far fewer workers than were needed with the “pilot plant” Callanan shuttle system, it was that system that provided packages for testing—both physical and for consumer application—and helped BIC refine the package before its roll-out to buyers last fall.
BIC U.S.A. went to its established suppliers on this project. It already purchased Alloyd blisters and heat-sealing equipment for other packages. And it also had a history of working with PromoEdge and its Machine Systems Div. that suggested the labeler and eventually wrote extra software and supplied a traversing arm that allowed the label head to shift between the label positions on the five- and 10-packs.
“There were two major requirements for the labeler,” says Tom Buchta of PromoEdge Machine Systems. “Most important was that it had to be radio-frequency resistant.” Since the labeler is relatively close to the RF generator, the wiring of the labeler had to be shielded so the radio waves wouldn’t affect it, he says. Unless the wiring was protected, the RF generator could trigger false signals at the labeler, Buchta points out. The second requirement was that the machine be CE-certified for the European marketplace.
Along with providing some extra programming to safeguard label application, PromoEdge helped the plant identify European companies that could help provide service.