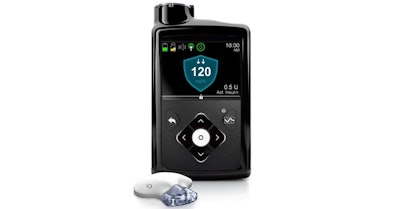
A May 2nd USA Today article contained good news for the 30 million Americans affected by diabetes. A new artificial pancreas is here to replace lancets, test strips and syringes. The MiniMed 670G is a closed loop hybrid system from Medtronic that continually self-adjusts to manage sugar levels based on the activity of its wearer, similar to how a Nest thermostat manages the climate in your home.
The device is only intended for type 1 diabetics whose pancreases produce little or no insulin. The device features a color screen that’s readable day or night, and it’s waterproof so it can be worn during underwater activities like swimming or snorkeling. The device was approved by the FDA in September of 2016, and is available now.