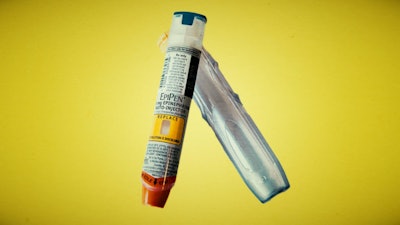
When an emergency strikes, the last thing you want to learn is that your expensive lifeline isn’t working. According to a recent article from STAT, that was the case for two users of Mylan’s EpiPen that led to the recall of over 80,000 units. Mylan released a statement that didn’t specify whether anyone was injured as a result of the failure, but a spokesperson noted that both individuals were able to use different EpiPens to relieve symptoms.
The recall includes devices that may contain a “defective part” that “may result in the device failing to activate or requiring increased force to activate.” The affected EpiPens were distributed in Australia, New Zealand, Japan, a handful of European countries, but not in the United States. The potentially defective injectors expire in April; patients can trade in their recalled EpiPen for a new one at no cost. The article also features ran interesting video explaining how EpiPens work.