Spinal implants and instruments typically arrive at a hospital or clinic in metal trays, without any sterile barrier system to protect them against pathogens. As such, they require medical facilities to sterilize them prior to use. Combine that with the growth of surgery centers and the need for facilities to better manage their purchasing and operational costs, and it becomes evident that the timing is perfect for packaging to step up to the plate and address these medical device sterilization challenges. (See Part I of this story.)
Why do some devices arrive at the hospital or clinic already sterilized while traditional spinal instruments and implants arrive in hospitals in metal trays that require sterilization by the hospital?
For decades, hospitals have been tethered to the practice of sterilizing reused medical devices at their facilities because of the inertia of the medical device industry. Historically, medical device manufacturers have failed to innovate beyond a reusable model using metal trays because of the ease of passing the work of sterilization onto the hospital. Hospitals had no choice but to use the kinds of surgical systems available on the market.
It is far more expensive for hospitals to sterilize metal trays before each procedure than to use a sterile packaged, single-use system. Xenco Medical does not require the hospital to pay for any of its instruments, only for the implant. This allows hospitals and ambulatory surgery centers to pay the same upfront cost on implants while savings thousands of dollars through eliminating sterilization costs and boosting turnover times. It was this critical gap that fueled Xenco Medical to develop the first single-use spinal systems made from a composite polymer instead of metal.
Describe how Xenco Medical products are packaged?
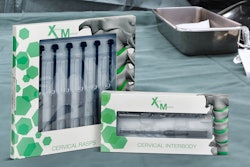
Xenco Medical instruments and implants come in a wide variety of sizing ranging from small packages designed to house individual pedicle screws to large packages that house entire sets of large surgical instruments. All of these packages consist of PETG trays that are heat-sealed with Tyvek® lids. We’ve continued to use PETG trays and Tyvek lids since the release of our first product because of their efficacy as a sterile barrier, their ability to withstand gamma irradiation, and their biocompatibility properties.
For the sealing of our packages, we use Belco Packaging Systems equipment. After gamma irradiation, a Harwell dosimeter is used to confirm that our products have sufficiently undergone gamma irradiation sterilization. For the printing of our labels, we use label printers from Zebra.
Implants are pre-attached to each delivery instrument and both are packaged into a sealed primary tray, which is then placed in a sealed secondary tray to provide optimal protection. Both are then placed inside of an identifying carton.
Following packaging, do all Xenco products then go through sterilization?
All Xenco Medical products are sterilized using gamma sterilization as that method is well-suited for single-use devices. The use of gamma sterilization is dependent on a packaging material’s ability to withstand that form of radiation. Gamma radiation uses cobalt 60, a radioactive isotope capable of penetrating sealed packages and killing microorganisms. This allows gamma radiation to be effective even when a surgical device is in its final package. We outsource our gamma sterilization process.
What are the logistics advantages for hospitals using Xenco products?
Hospitals and ambulatory surgery centers have reaped numerous logistical benefits by converting to Xenco’s single-use model. Beyond saving $850 to $950 per surgery through elimination of the costly sterilization process, our single-use systems have had an impact on turnover rates at both hospitals and ambulatory surgery centers. By eliminating a minimum 3.5-hr wait time between surgeries to sterilize and process the surgical systems, the Xenco devices allow centers to increase their number of surgeries and maximize the healthcare impact they have on their community.
For instance, research on surgery times has found that the average time for a two-level Anterior Cervical discectomy and Fusion, including anesthesia, is 2.3 hr. Our sterile-packaged system allows smaller centers to perform an additional procedure each day.
Following sterilization, Xenco products have a five-year shelf life. They are distributed throughout the U.S. and have been well-received by healthcare facilities using their products, by helping to reduce the overall sterilization volume, eliminating the autoclave process, and allowing additional surgeries to be performed at these facilities.