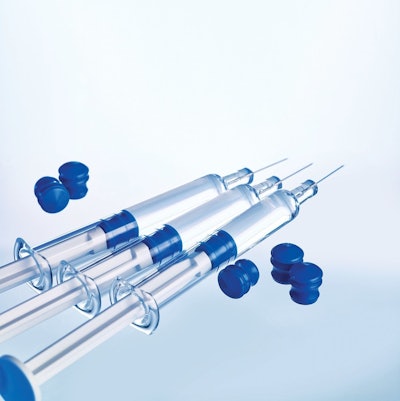
W.L. Gore & Associates, Inc. ("Gore"), a global materials science company, introduced the GORE™ ImproJect™ Plunger, which is intended for use in non-siliconized (bare glass), pre-filled syringe barrels.
Conventional plungers for pre-filled glass syringes are designed to work with lubricated barrels where silicone often acts as the lubricant between plunger and syringe during drug administration and provides a seal between plunger and syringe. However, silicone has been shown to potentially interact with sensitive biologics possibly leading to particulation and aggregation.
Over time, the measurable migration of silicone can also cause changes in break and glide force, which may impact injection performance and affect patient comfort.
By eliminating silicone from the barrel and the plunger of a pre-filled syringe, the ImproJectPlunger may enable more challenging sensitive biologics to be administered in a pre-filled syringe, a practice that maynot have been an option with traditional siliconized pre-filled syringe systems.
The proprietary fluoropolymer barrier supports compatibility with drug product as demonstrated in biocompatibility and extractables testing results. Silicone oil is not a raw material and is not used in the manufacture of the plungers.